Newborn Screening Notices
DSHS Laboratory Services Section – Notice to Public
Sign-up to receive automatic notices via email.
United States Postal Service (USPS)
Newborn Screening Specimen Shipping Issues
The Texas Department of State Health Services (DSHS) has received multiple reports from healthcare providers of USPS postal workers denying shipment of dried blood spots for newborn screening. These specimens are not subject to regulation under USPS Publication 52, Section 346.234d, meaning they are not regulated as Division 6.2 infectious substances, and you can mail them if they meet packaging requirements. USPS Packing Instructions 6G state that you must enclose the primary specimen in a secondary container.
Packaging Requirements:
- Allow specimens to dry for a minimum of three hours.
- Cover the specimen with the attached flap, which serves as the secondary container and bears the international biohazard symbol for USPS shipping regulations.
- Place the specimen in a blue diamond envelope provided by DSHS or a similar protective envelope. This envelope serves as the outer shipping container in compliance with USPS shipping regulations.
Recommended Actions:
- Inform USPS Staff: When mailing these specimens, provide a clear indication that the package contains dried blood spots as defined in USPS Publication 52, Section 346.234d and packaged according to USPS Packing Instruction 6G.
- Carry Documentation: It may be helpful to have a printed copy of this document or the relevant regulation to provide clarification if needed.
- Ask to speak to the Postmaster if issues persist.
The DSHS Laboratory is actively collaborating with USPS to resolve this matter and ensure efficient processing of your specimen shipments.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email: NewbornScreeningLab@dshs.texas.gov
Posted March 5, 2025
DSHS Laboratory Building Maintenance: Reference Laboratory to Process Newborn Screening Specimens
The Texas Department of State Health Services (DSHS) Public Health Laboratory in Austin, Texas, is undergoing building updates to prevent unexpected shutdowns. During this time, the Laboratory will have a series of planned weekend shutdowns.
To ensure timely newborn screening, DSHS has partnered with the reference laboratory Revvity Omics, which meets regulatory testing requirements.
Key Updates for Submitters:
- Submitters will continue to receive result reports from DSHS except in a different format.
- Reports will include a cover page with demographics and submitter information.
- Attached to the cover page will be the reference laboratory report.
- Submitters will receive results via mail, fax, or web application as usual.
- Submitters do not need to contact the reference laboratory for results.
- Results will be available through the Texas Newborn Screening Web Application.
- DSHS Clinical Care Coordination will continue follow-up on screen-positive results.
Electronic (HL7) Ordering and Reporting:
- Normal Results: Reported via HL7, as usual.
- Non–normal Results:
- Will include an out-of-range/abnormal determination and an abnormal flag.
- The result note will refer to the physical report for details.
- A physical report, including reference laboratory results, will get mailed to the submitter and be available via the Texas NBS Web Application.
Newborn screening is time sensitive. Delayed submissions can impact infant health outcomes. Please continue sending specimens promptly to ensure timely testing and follow-up care.
Thank you for your dedication to newborn screening and the health of Texas newborns.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted February 13, 2025
DSHS Austin Laboratory Open Regular Business Hours - Wednesday, January 22, 2025
The DSHS Austin Laboratory will return to normal operations Wednesday, January 22, 2025.
We understand that some areas may still be negatively affected by cold and icy conditions so please check with your courier for local service alerts. Courier services may experience delays due to prolonged cold or hazardous conditions.
Received specimens will be tested as soon as possible, and backlogs will be resolved during business hours.
-
Please Note: Specimens received outside of their acceptability window will be unsatisfactory for testing.
Questions? Contact the DSHS Austin Laboratory:
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Clinical Chemistry General Inquiries: 512-776-6236
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Environmental Chemistry Unit: EnvSciAdmin@dshs.texas.gov
Microbiology Unit: Lab.Microbiology@dshs.texas.gov
Microbiology General Inquiries: 512-776-7599
Newborn Screening General Inquiries: 512-776-7333
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Posted January 21, 2025
DSHS Austin Laboratory Closure Due to Inclement Weather - January 21, 2025
Inclement weather in Austin and other parts of Texas on Tuesday, January 21, 2025, may cause testing delays at the Laboratory.
-
The DSHS Austin Microbiology Unit and Environmental Sciences Laboratory will be closed on
Tuesday, January 21, 2025, due to inclement weather and hazardous road conditions. -
The Newborn Screening Laboratory will perform limited testing during that time.
-
Continue to ship all specimens per regular guidelines.
-
Due to the potential for shipping delays, please transfer serum for glucose and lipid testing from SST tubes into new tubes and freeze them. Then ship them only after weather conditions improve.
-
-
Courier services may experience delays due to the weather.
-
Please check with your courier for local service alerts.
-
-
All specimens will be processed as soon as possible after resumption of normal operations.
-
Note that specimens received outside of their acceptability window will be unsatisfactory for testing.
-
-
The Laboratory will work at maximum capacity to resolve any specimen backlog.
The DSHS Austin Laboratory appreciates the dedication and commitment of healthcare providers to keep Texans healthy.
Contact the DSHS Austin Laboratory:
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Clinical Chemistry General Inquiries: 512-776-6236
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Environmental Chemistry Unit: EnvSciAdmin@dshs.texas.gov
Microbiology Unit: Lab.Microbiology@dshs.texas.gov
Microbiology General Inquiries: 512-776-7599
Newborn Screening General Inquiries: 512-776-7333
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Posted January 20, 2025
Newborn Screening Guidance During Inclement Weather
As you prepare for possible winter weather, please remember:
- Do Not Delay Newborn Screening (NBS) Specimen Collection.
- Collect 1st screens at 24-48 hours of age.
- Collect 2nd screens between 7-14 days of age, a minimum of 168 hours.
- Ensure the parent/guardian contact information will be valid throughout any potential relocation.
- For patient transfers - Include newborn screening specimen collection status in transfer documentation. Notify receiving facility of specimen collection need if not collected before transfer.
- Allow NBS specimens to dry a minimum of 3 hours.
- DO NOT put specimens in air-tight sealed containers.
- Ship As Soon As Possible.
- If you use courier services, contact the courier for extra information about your location.
- During courier delays store dried specimens at room temperature in a dry location.
- If using USPS, walk your specimens inside the postal location. Do not drop specimens in the blue mailboxes outside.
Ensuring samples reach the lab in good condition helps provide accurate screening test results.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted January 08, 2025
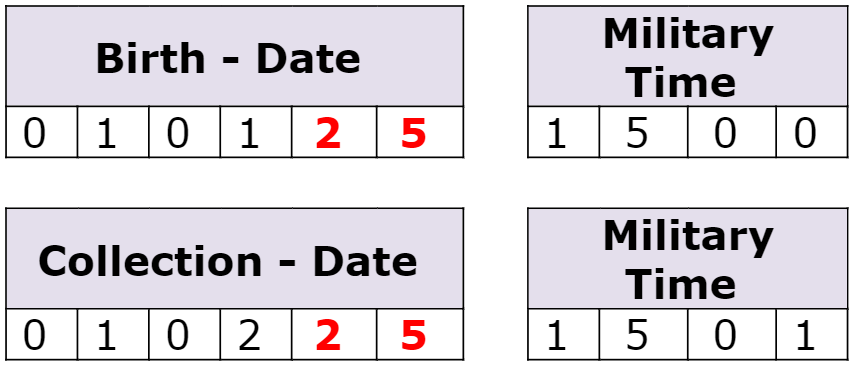
2025 Is Here
Incorrect dates DELAY specimen testing and can cause a specimen to be UNSATISFACTORY for testing.
To Avoid Delays:
- Ensure dates (including year) are complete and accurate.
- Assign staff to double-check all specimen information is:
- Complete
- Accurate
- Legible
- Respond to The Department of State Health Services (DSHS) request for clarifying information.
- Specimens are unsatisfactory for testing if information is not received by 1:00 p.m. the next business day.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or for local calls: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted January 02, 2025
Newborn Screening (NBS) for X-Linked Adrenoleukodystrophy (X-ALD) has Resumed Testing for all Specimens
With the resolution of instrument issues, DSHS has resumed testing X-ALD for all specimens.
- Regular testing for X-ALD has resumed.
- Revised reports with final X-ALD screening results are being sent for affected specimens originally reported as ‘Test Pending. Final report with results for this disorder to follow’.
- For submitters receiving electronic results, initial and revised results will transmit as usual for all affected specimens.
- For submitters who receive results by mail or by fax, you will receive your revised results by mail.
- Submitters who receive their results online will also get a copy by mail.
The DSHS Newborn Screening Program appreciates the dedication and commitment of healthcare providers to ensure the best possible outcomes for Texas newborns.
Questions?
Contact Newborn Screening Laboratory:
Phone: 1 (888) 963-7111 ext. 7333 or 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted December 23, 2024
DSHS Newborn Screening Laboratory Holiday Closure Reminder
Christmas Closure: Tuesday, December 24 and Wednesday, December 25, 2024, the Texas Department of State Health Services (DSHS) Newborn Screening (NBS) Laboratory will not be open, and no testing will occur. The NBS Laboratory will resume testing on Thursday, December 26, 2024.
New Year’s Day Closure: Wednesday, January 1, 2025, the Laboratory will not be open, and no testing will occur.
DO NOT Delay Collection or Shipment
- Ship dried NBS specimens within 24 hours of collection. If mail or courier services are unavailable, ship as soon as possible.
- DSHS recommends shipping NBS specimens using an overnight or trackable service like USPS Priority Mail, FedEx, or UPS.
- Per the FedEx website, FedEx will not be open on Wednesday, 12/25/2024 and Wednesday, 1/1/2025. FedEx will operate on a modified schedule December 23, 24, 26, 30 and 31. Please plan accordingly for your site.
Newborn Screening Supply Order Requests
- Standard orders received before noon Monday, 12/23/2024 will get processed and shipped via FedEx Ground.
- Priority overnight orders received by noon on Monday, 12/23/2024 will get processed and shipped via FedEx Priority Overnight.
- Orders received after noon Monday, 12/23/2024 through Thursday, 12/26/2024 will get filled Friday, 12/27/2024.
- Orders received on 1/1/2025 will get processed on 1/2/2025.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888-963-7111 ext. 7585 or for local calls: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Posted December 19, 2024
Newborn Screening for X-Linked Adrenoleukodystrophy (X-ALD)
Due to unexpected issues with testing instrumentation, DSHS is temporarily unable to complete X-ALD testing for some specimens.
- Affected specimens for the X-ALD disorder will report as “Test Pending”. A result reporting note will state, “Final report with results for this disorder to follow”.
- When testing is complete, DSHS will send a revised report with a final X-ALD screening result. The result will be on a regular DSHS result report.
- For submitters receiving electronic results, initial and revised results will transmit as usual for all affected specimens.
- DSHS will provide more communication on this issue and notify submitters when regular testing and reporting of X-ALD has resumed.
The DSHS Newborn Screening Program appreciates the dedication and commitment of healthcare providers to ensure the best possible outcomes for Texas newborns.
Questions?
Contact Newborn Screening Laboratory:
Phone: 1 (888) 963-7111 ext. 7333 or Locally: 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted December 17, 2024
2025 Will Soon be Here
Incorrect dates DELAY specimen testing and can cause a specimen to be UNSATISFACTORY for testing.
To Avoid Delays:
- Ensure dates (including year) are complete and accurate.
- Assign staff to double-check all specimen information is:
- Complete
- Accurate
- Legible
- Respond to The Department of State Health Services (DSHS) request for clarifying information.
- Specimens are unsatisfactory for testing if information is not received by 1:00 p.m. the next business day.

Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or for local calls: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted December 16, 2024
Avoid Holiday Shipping Delays
Newborn screening (NBS) specimens are unsatisfactory if received after 13 days of collection.
TIPS TO AVOID SPECIMEN REJECTION DURING HOLIDAYS:
- Allow NBS specimens to dry a minimum of three hours, per Clinical and Laboratory Standards Institute (CLSI).
- Ship dried specimens same day using an overnight or trackable service.
- Keep in mind that courier and mail services may have delays during the holidays.
- DO NOT hold specimens for bulk mailing or shipping.
- Ensure all specimens have the correct name, date of birth, and date of collection before shipping. Incorrect or incomplete demographic information can lead to testing delays or rejection.
- Assign a staff person to double check all NBS specimens. Check for spot quality and all demographic information is complete before shipment.
- Ship to the correct address:
For Overnight/Courier Shipping (UPS, FedEx, etc):
Texas Department of State Health Services
Laboratory Services Section, MC 1947
1100 W. 49th Street
Austin, TX 78756-3199
For USPS Regular and Priority Mail:
Texas Department of State Health Services
Laboratory Services Section, MC 1947
PO Box 149341
Austin, TX 78714-9341
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888-963-7111 ext. 7585 or for local calls: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted December 9, 2024
DSHS Austin Newborn Screening Laboratory Thanksgiving Holiday Closure Reminder
On Thursday, November 28, 2024, the Texas Department of State Health Services (DSHS) Austin Newborn Screening (NBS) Laboratory will not be open, and no testing will occur. Please see below for more details:
-
The NBS Laboratory will resume testing on Friday, November 29, 2024.
-
DO NOT delay collection or shipment of NBS specimens.
-
Ship dried specimens within 24 hours of collection. If mail or courier services are unavailable, ship as soon as possible.
-
DSHS recommends shipping specimens using an overnight or trackable service like USPS Priority Mail, FedEx, or UPS.
-
Per the FedEx website, FedEx will not be open on Thursday, 11/28/2024. FedEx will operate on a modified schedule November 27, 29, and 30th. Please plan accordingly for your site.
Newborn Screening Supply Order Requests
-
Standard orders received before noon Wednesday, 11/27/2024 will get processed and shipped via FedEx Ground.
-
Priority overnight orders received on Wednesday, 11/27/2024 will get processed and shipped via FedEx Priority Overnight.
-
Orders received after noon Wednesday, 11/27/2024 through Sunday, 12/01/2024 will get filled Monday, 12/02/2024.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or for local calls: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Posted November 21, 2024
Format of Newborn Screening Results Reports Has Changed
On the afternoon of November 4, 2024, the Texas Department of State Health Services Laboratory implemented format changes to the newborn screening result report statements. Going forward, all results will be in the new format.
A summary of the format changes is available in a previous notice. That notice is accessible here: Newborn Screening Notices | Texas DSHS
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted November 14, 2024
DSHS Austin Laboratory Thanksgiving Holiday Closure Reminder
With the exception of Newborn Screening and Rabies testing, the DSHS Austin Laboratory will be closed, and no testing will occur Thursday, 11/28/2024 through Saturday, 11/30/2024. The DSHS Laboratory is completely closed on Sundays. Testing services will resume as normal, Monday, 12/02/2024.
See below for more details on selected testing.
Newborn Screening (NBS)
-
The Newborn Screening Laboratory will be closed Thanksgiving Day, Thursday 11/28/2024 and will resume testing on Friday, 11/29/2024.
-
DO NOT DELAY collection or shipment of newborn screening specimens.
-
Ship dried specimens within 24 hours of collection. If mail or courier services are unavailable, ship as soon as possible.
-
DSHS recommends shipping specimens using an overnight or trackable service like USPS Priority Mail, FedEx, and UPS.
Clinical Chemistry
-
Total hemoglobin, lead, glucose, and lipid/cholesterol specimens must be received by Wednesday, 11/27/2024 to complete testing before the holiday closure.
-
DO NOT ship cholesterol, lipid profile, or glucose specimens on Fridays or on the day before a state-observed holiday.
Microbiology
Rabies Specimens
-
Rabies specimens received after 11:00 a.m. Wednesday, 11/27/2024 will be tested Friday, 11/29/2024. Submitters will be notified of positive results by 12:00 p.m. Friday.
All other results will be available Monday 12/02/2024.
-
Emergency testing will be available Friday, 11/29/2024 and Saturday, 11/30/2024 only by prior arrangement with Rabies testing staff. Call 512-776-7595 before 4:00 p.m. Wednesday, 11/27/2024 to discuss.
Specimen receipt must be guaranteed by 8:00 a.m. the morning of testing. Preliminary results will be called out as soon as emergency testing is completed. Final results will be available Monday 12/02/2024.
-
All other specimens will be tested on Monday, 12/02/2024, and results will be available by 5:00 p.m.
Tuberculosis (TB) Specimens
-
Do not ship specimens for delivery between Thursday, 11/28/2024 and Saturday, 11/30/2024.
Refrigerate specimens collected during this period until shipping is possible.
-
Ensure critical AFB Smear and NAA for M. tuberculosis specimens are delivered by 8:30 a.m. Wednesday, 11/27/2024.
-
Call 512-776-7342 before 9:00 a.m. on Wednesday, 11/27/2024 for expedited test requests.
Candida auris Swab Specimens
-
Do not collect or ship swabs for delivery from Tuesday, 11/26/2024 to Saturday, 11/30/2024.
-
Please remember that swabs must be received within 72 hours of collection.
Carbapenem Resistant Organisms Swab Specimens
-
Do not collect or ship swabs from Tuesday, 11/26/2024 to Saturday, 11/30/2024.
Neisseria gonorrhoeae Antimicrobial Susceptibility Testing Specimens
-
Do not collect or ship swabs from Tuesday, 11/26/2024 to Saturday, 11/30/2024.
Bacteriology Water Samples
-
Water samples will not be accepted at DSHS Laboratory from Wednesday, 11/27/2024 to Saturday, 11/30/2024.
All Other Microbiology Specimens or Samples
-
Do not ship specimens for delivery between Thursday, 11/28/2024 and Sunday, 12/01/2024. Store specimens collected during this time at an appropriate temperature (e.g. -70°C or below for clinical virology samples).
-
No testing will be performed from Thursday, 11/28/2024 to Sunday, 12/01/2024.
Ordering Laboratory Supplies
Newborn Screening Medicaid/Paid Card Order Requests
-
Priority overnight shipping requests received on Wednesday, 11/27/2024 will be processed and shipped via FedEx Priority Overnight.
-
All requests received before noon Wednesday, 11/27/2024 will be processed and shipped via FedEx Ground.
-
Requests received after noon Wednesday, 11/27/2024 through Saturday, 11/30/2024 will be sent Monday, 12/02/2024.
Texas Health Steps/PKU/TB/HIV/Courier Supply Order Requests
-
Requests received before 9:00 a.m. on Wednesday, 11/27/2024 will be audited, issued, and shipped via FedEx Ground Wednesday afternoon, 11/27/2024.
-
Requests received after 9:00 a.m. on Wednesday, 11/27/2024 through Sunday, 12/01/2024 will be audited, issued, and shipped on Monday, 12/02/2024 via FedEx Ground.
*** Per FedEx website, FedEx Ground will be closed Thursday, 11/28/2024 but will deliver packages Friday, 11/29/2024.***
Questions?
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Clinical Chemistry General Inquiries: 512-776-6236
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Environmental Chemistry Unit: EnvSciAdmin@dshs.texas.gov
Microbiology Unit: Lab.Microbiology@dshs.texas.gov
Microbiology General Inquiries: 512-776-7599
Newborn Screening General Inquiries: 512-776-7333
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Posted November 14, 2024
Changes to the Newborn Screening Result Reports
In early November, the Texas Department of State Health Services (DSHS) Laboratory will change the Newborn Screening (NBS) Result Reports. Examples of the updated NBS Result Reports are available for your review. Listed below is a summary of the changes to the report format.
Patient Information Changes:
- Condensed patient demographic and specimen information into three columns.
- Include some shortened field names to save space.
- No longer include the following fields:
- Mother’s Address
- Race/Ethnicity
- Test [i.e., 1st Test (under 7 days), 2nd Test (7 days or over)]
Result Information Changes:
- No longer display analyte and analyte result columns in the result table.
- However, analyte results will remain in the screening result notes for abnormal screens.
Testing Information Changes:
- No longer include a list of each NBS disorder screened at the bottom of the result report.
- Incorporate a web address and QR code that links to the NBS Disorders Screened webpage on the DSHS Laboratory NBS website.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888-963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted November 04, 2024
Meet the Heroes Behind Newborn Screening: Celebrating the Teams Who Safeguard Texas Babies’ Health
As we celebrate Newborn Screening Awareness Month, we want to take this opportunity to introduce you to the dedicated testing teams behind the scenes, working tirelessly to protect the health of Texas newborns. These teams play a crucial role in early detection, helping save lives and ensuring that babies have the best possible start. Visit our NBS Awareness Website to learn more about their important work.
Here’s a look at our amazing teams:
- Biotinidase Deficiency Screening Team: Using color-changing reactions, this team tests for biotinidase deficiency, ensuring that babies who cannot process biotin get the treatment they need to avoid serious health issues.
- Enzyme Immunoassay (EIA) Team: This team screens for critical disorders like galactosemia, congenital hypothyroidism, congenital adrenal hyperplasia, and cystic fibrosis using enzyme-linked immunosorbent assays (ELISA). Their early detection efforts are vital in preventing life-threatening complications.
- Hemoglobinopathy Team: Working with isoelectric focusing and high-performance liquid chromatography techniques, this team detects genetic blood disorders like sickle cell disease and other hemoglobin variants, helping to ensure early intervention for affected infants.
- Severe Combined Immunodeficiency (SCID) and Spinal Muscular Atrophy (SMA) Team: This team screens for both SCID and SMA using advanced real-time polymerase chain reaction methods, enabling early diagnosis and treatment of these life-threatening conditions.
- Mass Spectrometry and LC Mass Spectrometry Teams: With its expertise in screening for x-linked adrenoleukodystrophy and over 40 metabolic conditions, including amino acid, fatty acid oxidation, and organic acid disorders, these teams ensure the earliest possible detection of rare metabolic disorders.
- DNA Analysis Team: Performing molecular confirmatory testing for disorders like cystic fibrosis and galactosemia, this team ensures accurate screening results, helping healthcare providers make informed decisions for newborn care.
Every day, these teams actively commit to improving the health of Texas newborns and make a real difference. Thank you for supporting our mission and celebrating Newborn Screening Awareness Month with us! Let’s continue raising awareness and supporting newborn screening!
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted September 18, 2024
Critical Congenital Heart Disease – We Are Newborn Screening
September is Newborn Screening Awareness Month
Critical Congenital Heart Disease (CCHD) screening is a simple, non-invasive test designed to detect serious heart defects in newborns. CCHD is one of the leading causes of death in infants less than one-year-old. The Centers for Disease Control and Prevention (CDC) reports that about one in four babies born with a heart defect has a critical congenital heart defect. The Texas Health and Safety Code requires reporting of confirmed CCHD cases to the Texas Department of State Health Services.
The Special Projects Team coordinates CCHD duties. Visit We Are Newborn Screening | Texas DSHS to learn more about the specific roles these team members play in our CCHD activities.
For Questions Please Contact:
Newborn Screening Clinical Care Coordination
Phone: 512-776-3957
Toll-free: 800-252-8023 ext. 3957
Newborn@dshs.texas.gov
Posted September 16, 2024
We Are Newborn Screening – Meet the teams that keep us moving.
We would like to introduce you to the teams that form the backbone of the preanalytical process in Newborn Screening (NBS). Each of these teams work to ensure the NBS process gets off to a good start. Accurate and timely screening for newborns begins with the following teams:
- Lab Supply/Container Preparation Teams: maintain the availability of essential supplies needed for specimen collection and testing.
- NBS Check-In Team: manages the initial receipt and verification of all specimens.
- Specimen Logistics Team: ensures the smooth flow of specimens from collection to testing.
- Specimen Preparation: prepares the specimens for testing. Processes specimens into testing plates ensuring reliable NBS results.
We invite you to learn more about each team by visiting the 2024 NBS Awareness Month Webpage. You can see pictures of our amazing staff and their work areas.
For Questions Please Contact:
Newborn Screening Laboratory Educators:
Phone: 512-776-7585
Toll-free: 1-888-963-7111, ext. 7585
Fax: 512-776-7157
NewbornScreeningLab@dshs.texas.gov
Posted September 11, 2024
Texas Early Hearing Detection and Intervention (TEHDI) – We Are Newborn Screening
September is Newborn Screening Awareness Month
Did you know early intervention for hearing loss can make a world of a difference in a child’s development? The TEDHI Team promotes understanding of critical timelines and steps to manage hearing loss. Each year in Texas, about 500 babies are born deaf or hard of hearing. Hearing loss is the second most common birth defect in Texas.
We encourage you to take a moment to meet the team and learn about all the work they do to help Texas children. Together we can continue to support children’s development and well-being.
For Questions Please Contact:
TEHDI, Newborn Screening
Phone: 512-483-7417
Toll-free: 800-252-8023, ext. 6616
TEDHI@dshs.texas.gov
Posted September 9, 2024
Newborn Screening Guidance During Hurricane Season
As you prepare for possible bad weather, please remember:
- Do Not Delay Newborn Screening (NBS) Specimen Collection.
- Collect 1st screens at 24-48 hours of age.
- Collect 2nd screens between 7-14 days of age, a minimum of 168 hours.
- Ensure the parent/guardian contact information will be valid throughout any potential relocation.
- For patient transfers - Include newborn screening specimen collection status in transfer documentation. Notify receiving facility of specimen collection need if not collected before transfer.
- Allow NBS specimens to dry a minimum of 3 hours.
- DO NOT put specimens in air-tight sealed containers.
- Ship As Soon As Possible.
- If you use courier services, contact the courier for extra information about your location.
- During courier delays store dried specimens at room temperature in a dry location.
- Walk your specimens inside the postal location. Do not drop specimens in the blue mailboxes outside. Mailboxes become very hot, humid and can flood during high water events.
Ensuring samples reach the lab in good condition helps provide accurate screening test results.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted September 9, 2024
Together – We Are Newborn Screening September is Newborn Screening Awareness Month
It takes a team effort to manage a newborn screening (NBS) program as big as Texas. Together,
since 1965 we have made a difference in the lives of our youngest Texans. Texas is home to the
largest NBS program in the United States. This year, we are shining a spotlight on the incredible
teams working behind the scenes to ensure every baby born in Texas gets the care they need.
In 2023, we achieved remarkable milestones:
- Screened over 760,000 specimens from almost 400,000 babies for 55 disorders.
- Provided hearing screening for babies born in Texas.
- Screened newborns for critical congenital heart disease.
These accomplishments are a testament to the dedication and hard work of our teams. We
encourage you to take a moment to recognize your team and all the work they put into
making NBS successful in Texas. Together with our partners, we operate a Texas-sized NBS
program that gives babies the best possible start to a healthy life. Let’s continue to work
together to protect and support our youngest Texans.
For Questions Please Contact:
Newborn Screening Laboratory Educators:
Phone: 512-776-7585
Toll-free: 1-888-963-7111, ext. 7585
Fax: 512-776-7157
NewbornScreeningLab@dshs.texas.gov
Posted September 4, 2024
Newborn Screening Quality Improvement Hints
Caked, Clotted, or Layered Blood on Filter Paper
In 2023, over 2,102 newborn screening specimens were rejected because the blood was caked, clotted, or layered onto the filter paper. All these specimens required a recollection and caused critical delays in testing.
Why is caked, clotted, or layered specimens rejected?
The supplied newborn screening filter paper can hold a specific amount of blood. Caked, clotted, or layered blood specimens have too much blood. Specimens with too much blood can cause inaccurate results. These specimens will be unsatisfactory for testing.
Download a copy of the Quality Improvement Hint: Blood was caked, clotted, or layered.
View other NBS Healthcare Provider Resources.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted August 15, 2024
Changes to 2024 Newborn Screening Specimen Collection Kits
2024 newborn screening specimen collection kits will begin shipping soon. The new collection kits begin with serial numbers starting 24 (24XXXXXXX). The following are changes from previous kits.
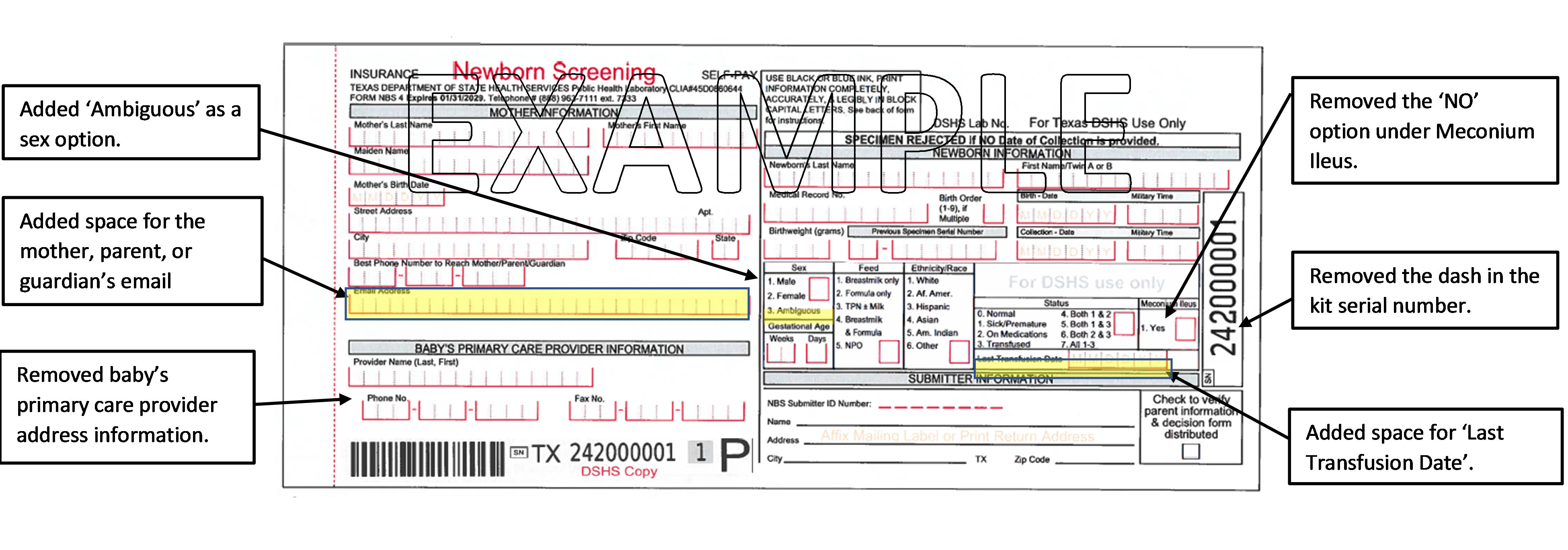
Please ensure all information is complete, accurate, and legible before shipping specimens to DSHS. Incorrect or missing information can cause:
- specimen rejection
- delayed testing
- incorrect test results and reports
Are older versions of the collection kit still acceptable?
Yes, all collection kits that have not expired are still acceptable. The expiration date is printed on all collection kits.
For Questions Please Contact:
Newborn Screening Laboratory
Phone: 512-776-7333
Toll-free: 1-888-963-7111 ext. 7333
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted August 12, 2024
DSHS Lab Web Application System Maintenance Scheduled Sunday, August 11, 9:00 a.m. to 11:00 a.m.
DSHS will be performing system maintenance 9:00 a.m. to 11:00 a.m. Sunday, August 11, 2024.
During this time, the DSHS Newborn Screening Web Application (Neometrics) and the DSHS Microbiology and Clinical Chemistry Web Application (Public Health Lab Online) may be unavailable.
Need help?
Contact DSHS Laboratory:
Phone: 1-888- 963-7111 ext. 7318 or Locally: 512-776-7318
Email: Labinfo@dshs.texas.gov
Posted August 9, 2024
Newborn Screening Guidance During Hurricane Season
As you prepare for possible bad weather, please remember:
- Do Not Delay Newborn Screening (NBS) Specimen Collection.
- Collect 1st screens at 24-48 hours of age.
- Collect 2nd screens between 7-14 days of age, a minimum of 168 hours.
- Ensure the parent/guardian contact information will be valid throughout any potential relocation.
- For patient transfers - Include newborn screening specimen collection status in transfer documentation. Notify receiving facility of specimen collection need if not collected before transfer.
- Allow NBS specimens to dry a minimum of 3 hours.
- DO NOT put specimens in air-tight sealed containers.
- Ship As Soon As Possible.
- If you use courier services, contact the courier for extra information about your location.
- During courier delays store dried specimens at room temperature in a dry location.
- Walk your specimens inside the postal location. Do not drop specimens in the blue mailboxes outside. Mailboxes become very hot, humid and can flood during high water events.
Ensuring samples reach the lab in good condition helps provide accurate screening test results.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted July 3, 2024
Texas Summer Heat Can Affect Specimen Quality
Shipping newborn screening (NBS) specimens during summer months can be a challenge. Heat and humidity can affect the stability of the specimens, causing inaccurate results. It is important to transport specimens at a controlled temperature, if possible.
Here are some key points to consider:
- Schedule mail pickups inside your location. Do not place specimens on outside of facility door for pickup.
- Walk your specimens inside the postal location. Do not drop specimens in the blue mailboxes outside. Mailboxes become very hot and humid.
- Never leave specimens inside a non-running closed automobile.
Ensuring samples reach the lab in good condition help provide accurate screening test results.
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted June 7, 2024
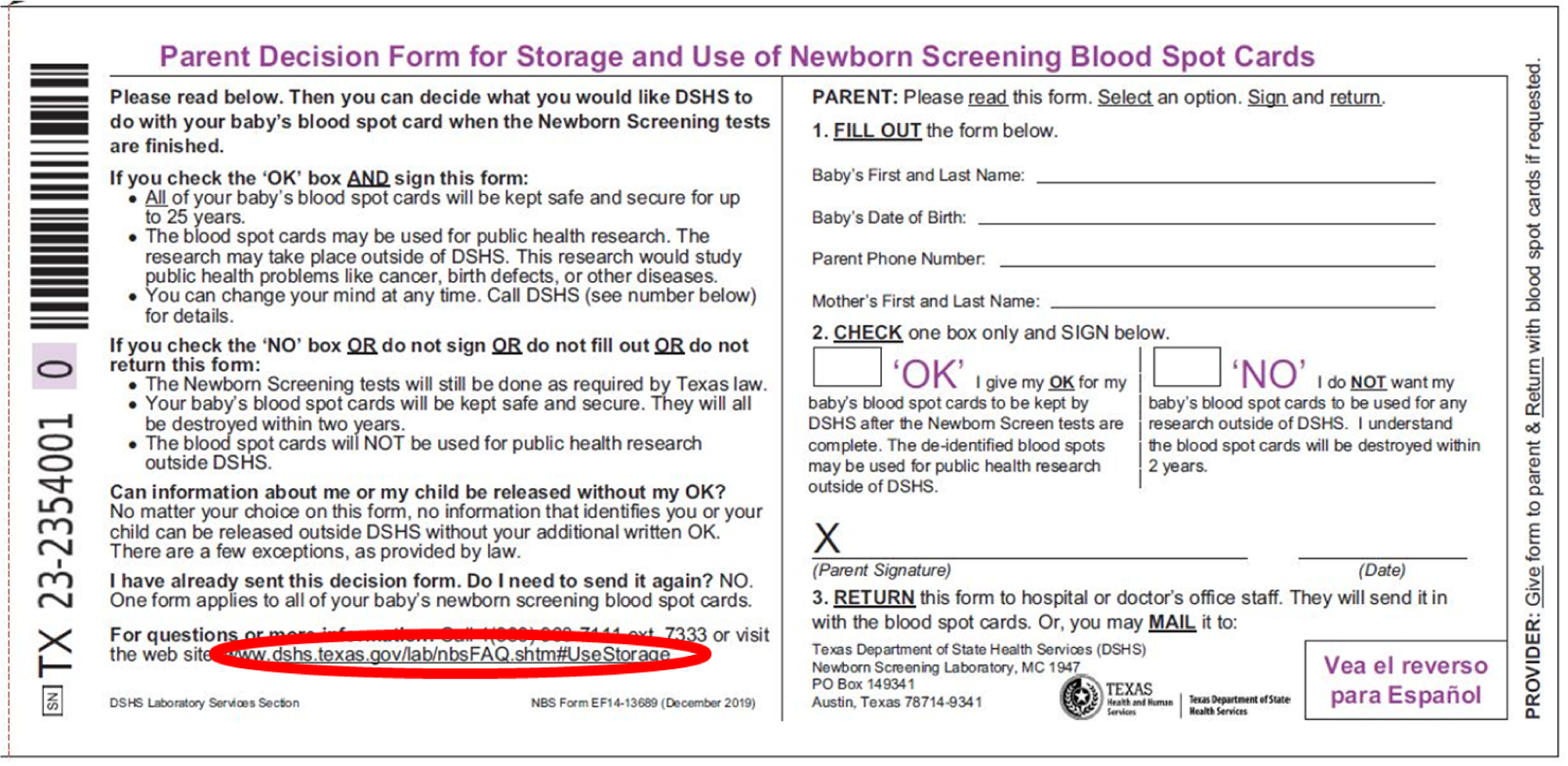
Correct Website to Parent Decision Form for Storage and Use of Newborn Screening Blood Spot Cards Information
The Texas Department of State Health Services (DSHS) migrated its website to a new platform in 2023. The move deactivated a website address listed on all the Newborn Screening (NBS) collection kits currently in use. The Parent Decision Form for Storage and Use of Newborn Screening Blood Spot Cards page now contains the wrong website address. See the image below “for questions or more information” section. The affected kits are those with serial numbers starting with 21-XXXXXXX, 22-XXXXXXX, and 23-XXXXXXX.
The correct website address to direct parents to Newborn Screening - Frequently Asked Questions.
This summer, we will begin distributing the 2024 NBS collection kits (24-XXXXXXX) with the correct website address.
For Questions Please Contact:
Newborn Screening Laboratory
Phone: 512-776-7333
Toll-free: 1-888-963-7111 ext. 7333
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted May 20, 2024
A message from Grace Kubin, Ph.D., Associate Commissioner, DSHS Public Health Laboratory Division
It’s that time of year again! Each year, the DSHS Austin Public Health Laboratory conducts a survey, open to all our customers, to provide important feedback on laboratory services. Your feedback is used by the laboratory to better understand your needs and identify gaps in service as part of the laboratory's commitment to continuous quality improvement. If you are interested to see the results of last year’s survey, you can find them here: 2023 Annual DSHS Laboratory Customer Service Survey Results Summary.
I encourage you to take the 2024 Public Health Laboratory Customer Service Survey. It should not take more than 10 minutes to complete. For questions or concerns, please send an email to: Labinfo@dshs.texas.gov.
2024 Public Health Laboratory Customer Service Survey
**Feedback will be collected until April 30, 2024**
Posted April 1, 2024
Newborn Screening Quality Improvement Hints Importance of Birthweight
DSHS Newborn Screening (NBS) Laboratory evaluates test results based on birthweight. Leaving the birthweight blank on the NBS demographic page may delay testing. Test results may not be accurate if the weight is wrong or not in grams.
Download a copy of the Quality Improvement Hint: Importance of Birthweight
View a weight conversion chart and other NBS Healthcare Provider Resources.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted March 22, 2024
Newborn Screening Laboratory Addresses for Receiving Specimens
The Texas Department of State Health Services (DSHS) Laboratory can receive both overnight shipments and regular United States Postal Service (USPS) mail deliveries. Submitters should be mindful of the time between collection, shipment, and delivery. DSHS recommends shipping specimens using an overnight or trackable service like USPS Priority Mail, FedEx, or UPS.
The clock starts ticking the day of collection, which equals day one. Newborn screening (NBS) specimens are unsatisfactory if received after 13 days of collection and will need a recollection. Best practice is to allow NBS specimens to dry a minimum of 3 hours, per Clinical and Laboratory Standards Institute (CLSI). Ship specimen same day using an overnight or trackable service.
For Overnight/Courier Shipping (UPS, FedEx, etc.):
Texas Department of State Health Services
Laboratory Services Section, MC 1947
1100 W. 49th Street
Austin, TX 78756-3199
For USPS Regular and Priority Mail:
Texas Department of State Health Services
Laboratory Services Section, MC 1947
PO Box 149341
Austin, TX 78714-9341
Need help?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted March 4, 2024
Clarification: Changes to Newborn Screening Cystic Fibrosis Testing and Result Reporting Statements Effective February 5, 2024
The Texas DSHS Newborn Screening (NBS) Laboratory updated and added new result codes for Cystic Fibrosis to improve fidelity in reporting by reducing the frequency of manual result entry. No changes were made to the DSHS CF DNA variant panel. For all existing CF DNA results, the CFTR mutation analyte result in the result grid of the report was updated to match the language in the result notes (variant instead of mutation per ACMG guidelines). Eighty new CFDNA result combinations that previously required free text entry were added to the list of results linked below. These updates went into effect on February 5, 2024.
View a full list of all Newborn Screening Laboratory Results.
Reminder: Review the result screening note(s) for all CF DNA results.
Questions?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted February 27, 2024
Changes to Newborn Screening Cystic Fibrosis Testing and Result Reporting Statements Effective February 5, 2024
The Texas DSHS Newborn Screening (NBS) Laboratory updated and added new result codes for Cystic Fibrosis to improve fidelity in reporting by reducing the frequency of manual result entry. For all existing CF DNA results, the CFTR mutation analyte result in the result grid of the report was updated to match the language in the result notes (variant instead of mutation per ACMG guidelines). Additionally, 80 new CF DNA results were added. These updates went into effect on February 5, 2024.
View a full list of all Newborn Screening Laboratory Results.
Reminder: Review the result screening note(s) for all CF DNA results.
Questions?
Contact DSHS Newborn Screening Laboratory:
Phone: 1-888- 963-7111 ext. 7585 or Locally: 512-776-7585
Email Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Posted February 20, 2024
2024 Is Here
Incorrect dates DELAY specimen testing and can cause a specimen to be UNSATISFACTORY for testing.
To Avoid Delays:
- Ensure dates (including year) are complete and accurate.
- Assign staff to double check that all specimen information is:
- Complete
- Accurate
- Legible
- Respond to DSHS requests for clarifying information immediately. If information is not received by 1:00 p.m. the next business day, the specimen will be deemed unsatisfactory for testing.


For questions or comments
Call toll free: 1-888-963-7111 ext. 7333 or for local calls 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted January 2, 2024
Cost of the DSHS Newborn Screening Kit will Increase January 1, 2024
Effective January 1, 2024, the cost of Private Pay Insurance/Self-Pay newborn screening (NBS) kits will increase by 8%, from $63.55 to $68.63. Insurance/Self-Pay NBS kits ordered on or after January 1, 2024, will be billed at the new price of $68.63.
The purpose of this increase will be to support increased costs for operations and is not for the addition of any new conditions to the Department of State Health Services newborn Screening panel.
View complete details, including the current Austin and South Texas DSHS Laboratory fee schedules.
For Questions Please Contact:
Newborn Screening Laboratory
Phone: 512-776-7333
Toll-free: 1-888-963-7111 ext. 7333
NewbornScreeningLab@dshs.texas.gov
Posted December 13, 2023
DSHS Newborn Screening Improvement to Primary Congenital Hypothyroidism Testing Methodology
The Department of State Health Services (DSHS) Newborn Screening (NBS) Program—in collaboration with the CDC, Texas endocrinology consultants, and national experts—conducted an improvement project to optimize screening for Primary Congenital Hypothyroidism (CH).
Goals of the project:
- Optimize screening algorithm for identification of CH and reduce the false positive rate and age at diagnosis.
- Reduce the time to provider notification for critically high TSH levels.
- Improve outcomes and reduce costs to the overall healthcare system.
On December 4, 2023, changes will be implemented to the initial screening CH testing methodology based on this project’s findings.
- Specimens collected before 7 days of life will be screened using Thyroid Stimulating Hormone (TSH). A subset of specimens with slightly elevated TSH levels will be retested for both TSH and Thyroxine (T4) levels.
- All specimens collected at 7 days of life or later will be screened using both Thyroxine (T4) and Thyroid Stimulating Hormone (TSH).
There will be no changes to result reporting statements, HL7 reporting or recommended follow-up activities at this time.
Where can I find more information on Primary Congenital Hypothyroidism (CH)?
Find more information at Congenital Hypothyroidism ACT sheet.
For Questions Please Contact:
Newborn Screening Laboratory
Phone: 512-776-7333
Toll-free: 1-888-963-7111 ext. 7333
NewbornScreeningLab@dshs.texas.gov
Posted December 04, 2023
2024 Will Soon be Here.
Incorrect dates DELAY specimen testing and can cause a specimen to be UNSATISFACTORY for testing.
To Avoid Delays:
- Ensure dates (including year) are complete and accurate.
- Assign staff to double check that all specimen information is:
- Complete
- Accurate
- Legible
- Respond to DSHS requests for clarifying information. Specimens with information not received by 1:00 p.m. the next business day will be deemed unsatisfactory for testing.
 For questions or comments:
For questions or comments:
Call toll free: 1-888-963-7111 ext. 7333 or for local calls 512-776-7333
Email: NewbornScreeningLab@dshs.state.tx.us
Posted December 01, 2023
Updates to the Login Process for NBS Web Application (Neometrics)
To improve data security, the number of incorrect logins attempts on the Newborn Screen (NBS) Web Application, Neometrics, prior to account lock has been reduced from five to three.
Texas Department of State Health Services (DSHS) security policy requires deactivation of accounts that have not been accessed within 90 days. Users will now receive an email providing notification of upcoming account deactivations due to non-use. This notice will instruct users to log into the NBS Web Application account to keep their accounts active.
Need help?
Contact Remote Lab Support:
Email: remotelabsupport@dshs.texas.gov
Posted October 05, 2023
Importance of Residual Blood Spot Consent
The Texas Department of State Health Services (DSHS) Newborn Screening Laboratory recommends five complete blood spots for the newborn screen. This ensures that there will be enough blood to complete all screening and retesting for any abnormal results. The blood that remains after all testing is complete is the residual blood spots. These blood spots have important uses for both DSHS and public health research.
After testing, DSHS keeps residual blood spots in a secure location for up to two years. By Texas law (Health & Safety Code Sec. 33.018 (b)-(c)), the residual blood spots are available for use during that time. Possible uses during the two-year time include:
- Ensuring DSHS newborn screening tests, equipment, and supplies are working.
- Developing new tests for newborn screening.
- Studying diseases that affect public health as allowed by law.
If the parent gives their "OK" on the Parent Decision Form for Storage and Use of Newborn Screening Blood Spots, the residual blood spots are safely stored for up to 25 years. The blood spots may be used for public health research outside of DSHS. The research would study public health problems like cancer, birth defects, and other diseases. Identifying information about the parent or child will not be released outside of DSHS without additional written consent.
Residual blood spots are vital to improve the health of current and future children in Texas and beyond. Research using dried blood spots helps improve screening tests for disorders in newborns and development of better treatments or cures.
For Questions Please Contact:
Newborn Screening Laboratory Educators
Phone: 512-776-7585
Fax: 512-776-7157
Toll-free: 1-888-963-7111 ext. 7585
NewbornScreeningLab@dshs.texas.gov
Posted September 27, 2023
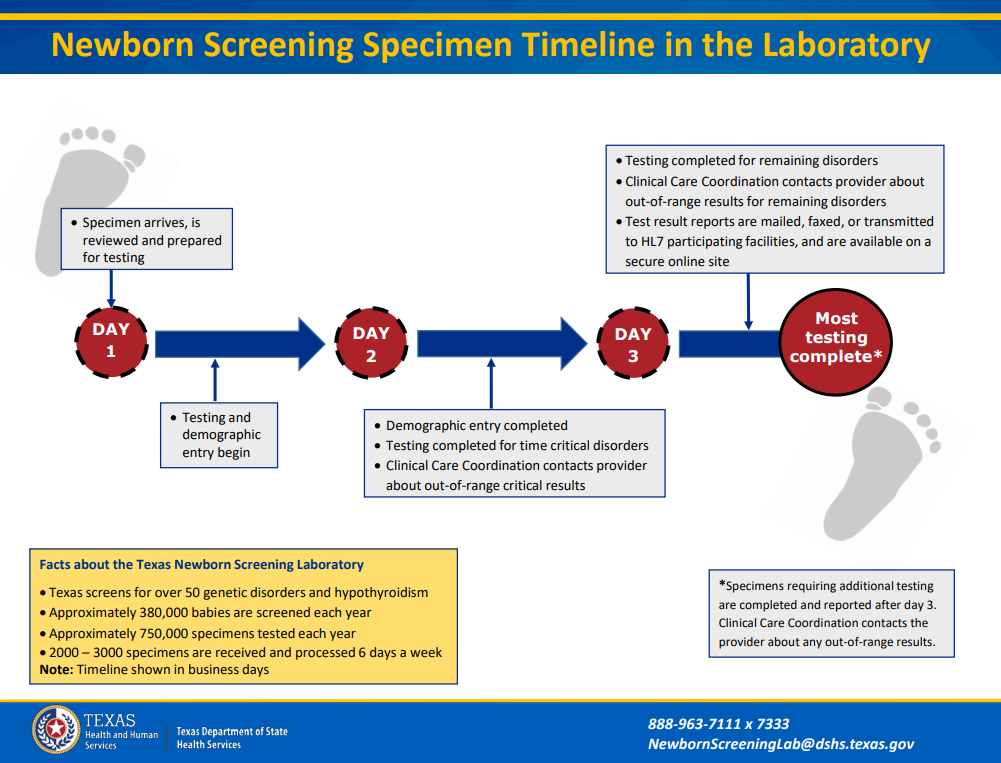
Newborn Screening Timeliness; a Texas-Sized Task
The saying “Everything is Bigger in Texas” applies to the Texas Newborn Screening (NBS) Program. The Texas Department of State Health Services (DSHS) Laboratory tests over 750,000 NBS specimens per year.
The babies of Texas, who may be born with time-critical conditions, are counting on us to get the results to their healthcare provider. The clock to timely reporting starts ticking as soon as submitters collect a NBS specimen. National recommendations state that first screen specimens should arrive at the DSHS Laboratory within 24 hours after collection.
During 2022, our percentage for reporting time-critical presumptive positive results within 5 days of life was 40.9%, far below the national goal of 95%.
To improve time-critical reporting submitters should:
- Collect a quality specimen at 24 hours of life.
- Let the specimen dry for at least 3 hours.
- Ship the specimen, via overnight courier, to the DSHS Lab as soon as possible.
The review, preparation, and testing of a newborn specimen begins the day it arrives at the DSHS Lab. The DSHS Lab processes 2,000-3,000 specimens a day, 6 days a week. Most testing is completed on a timeline spanning 2 to 3 business days after specimen receipt. Once testing is complete, results are sent to DSHS NBS follow-up staff who call the infant’s doctor, ensuring the baby gets follow-up care without delay. There is always room for improvement and increasing timely reporting is important for the health of Texas babies. Newborn Screening Educators are available to provide education and tips on NBS process improvements.
For Questions Please Contact:
Newborn Screening Laboratory Educators
Phone: 512-776-7585
Fax: 512-776-7157
Toll-free: 1-888-963-7111 ext. 7585
NewbornScreeningLab@dshs.texas.gov
Posted September 20, 2023
Department of State Health Services Public Health Laboratory Division has revised Newborn Screening Laboratory Testing Fees
The Department of State Health Services (DSHS) Public Health Laboratory Division has revised newborn screening laboratory testing fees to meet business needs. This revision takes effect January 1, 2024.
The cost of PAID newborn screening (NBS) kits will be increased by 8% (from $63.55 to $68.63). Paid NBS kits, ordered on or after January 1, 2024, would be billed at the new price of $68.63. To view complete details, visit the DSHS Laboratory’s Fee Schedule webpage.
Posted September 11, 2023
Importance of Collecting Two Newborn Screening Tests
The Texas Department of State Health Services implemented a two-screen system in 1983 to maximize the effectiveness of testing for disorders on the Texas Newborn Screening Panel. Every baby born in Texas gets two newborn screening tests to screen for 55 rare disorders.
The first newborn screen is collected 24-48 hours after birth and the second at 7-14 days of life. Combined, the two screens help identify disorders at the earliest possible opportunity. In Texas, the second screen routinely detects babies at risk for severe medical complications. These babies sometimes have a normal first screen.
The timing of collecting newborn screening is important. After a baby is born, their physiology changes in the first days and weeks of life. For this reason, some disorders can only be detected immediately after birth or one to two weeks after birth when the second screen is collected. Some disorders, like cystic fibrosis, rely on information from both the first and second newborn screen to better determine which babies need extra testing and referral to a specialist.
The goal of the Texas Newborn Screening Program is to identify babies who have these devastating diseases before symptoms arise so that they can receive appropriate treatment and lead productive and healthy lives. Having a second screen improves medical provider’s ability to identify children with disorders who would have otherwise gone undetected.
For Questions Please Contact:
Newborn Screening Laboratory Educators
Phone: 512-776-7585
Fax: 512-776-7157
Toll-free: 1-888-963-7111 ext. 7585
NewbornScreeningLab@dshs.texas.gov
Posted September 11, 2023
September is National Newborn Screening Awareness Month

The Texas Department of State Health Services Newborn Screening Program began in 1965 with statewide screening for Phenylketonuria (PKU). Since then, the program has grown, and the State of Texas is now screening for 55 disorders.
Because Newborn Screening began with one test looking for PKU, many began calling the Newborn Screen Test the “PKU test,” a term which is now outdated and misleading. PKU is only one of the many disorders screened for on the Newborn Screening Test. Telling a caregiver, provider, or other attending staff the newborn has an abnormal “PKU test” can be confusing and result in incorrect follow-up testing and treatment.
We challenge YOU to become aware and make a conscious effort to remove the confusing label of “PKU” or “PKU test” when talking about the Newborn Screening Test.
The Evolution of Texas Newborn Screening timeline on our website offers a glimpse into how the Newborn Screening program has grown from piloting and testing for only PKU in the 1960s to 55 disorders today.
For Questions Please Contact:
Newborn Screening Laboratory Educators
Phone: 512-776-7585
Fax: 512-776-7157
Toll-free: 1-888-963-7111 ext. 7585
NewbornScreeningLab@dshs.texas.gov
Posted September 5, 2023
2022 Texas Newborn Screening Annual Report
TOGETHER in 2022 we:
- Screened about 394,000 babies and over 761,000 newborn screening (NBS) specimens.
- Identified 1,130 babies born in 2022 (to date) affected by one of the NBS disorders.
KEEP UP THE GREAT WORK!
The 2022 Texas Newborn Screening by the Numbers Report provides:
- Annual Texas NBS statistics
- Texas’ status on achieving the ACHDNC* recommended timeliness goals. (Goal is 95%)
- View‘Timely Collection and Submission of NBS Specimens’ for information on meeting ACHDNC and Texas timeliness goals.
|
ACHDNC* Recommended |
Texas |
|---|---|
|
Initial NBS specimen collection: First NBS screens should be collected within the appropriate time frame for the newborn’s condition but no later than 48 hours after birth. |
97.3% |
|
Transportation of initial NBS specimens: First NBS screens should be received at the laboratory as soon as possible; ideally within 24 hours of collection. Specimens received within 24 hours of collection. |
18.1% |
|
Transportation of initial NBS specimens: First NBS screens should be received at the laboratory as soon as possible. Specimens received within 48 hours of collection. |
63.4% |
|
Reporting of initial screen presumptive positive time critical condition** results: Results should be reported to healthcare provider as soon as possible, but no later than five days of life. |
40.9% |
|
Reporting of initial screen presumptive positive time sensitive condition** results: Results should be reported as soon as possible, but no later than seven days of life. |
83.7% |
|
Completion of all initial screen NBS tests within seven days of life with results reported to the healthcare provider as soon as possible. |
83.4% |
* United States Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC)
**List of NBS Disorders by Time Criticality
For general questions or comments:
Call toll-free: 1-888-963-7111 ext. 7333 or for local calls 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted August 24, 2023
Request of Sickle Cell Results for Student-Athletes
NCAA Policy requires sickle cell test results for all student-athletes as of August 2022.
The Texas newborn screening panel includes testing for sickle cell trait.
As a Healthcare Provider, you can do one of the following:
- Contact DSHS Laboratory Reporting Department at 512-776-7578, on behalf of the patient, to request their Newborn Screening report. This is the fastest and most efficient way for the student-athlete to obtain their sickle cell results, or
- Fax the request to DSHS Laboratory at 512-776-7533, or
- Direct the student-athlete to the DSHS webpage, “How Patients Can Request Copies of Test Results”, https://www.dshs.texas.gov/lab/patientresults.aspx. The athlete will need to download the Authorization to Release Laboratory Results Form. All fields are required. They will need to fax, mail, or securely email the completed Authorization to Release Laboratory Results Form and a copy of their current and valid government-issued photo ID to DSHS Laboratory.
- DSHS Lab Reporting Department fax number: 512-776-7533
- DSHS Laboratory Reporting Group, MC 1947 P.O. Box 149347 Austin, TX 78714-9347
- Email: Labinfo@dshs.texas.gov
For general questions or comments:
Call toll-free: 1-888-963-7111 ext. 7333 or for local calls 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted July 3, 2023
2023 Annual DSHS Laboratory Customer Service Survey
A message from Grace Kubin, Ph.D., Director, DSHS Laboratory Services Section.
Your feedback is very important! Each year, the DSHS Laboratory initiates a survey open to all our customers to provide important feedback on laboratory services. Your feedback is used by the laboratory to better understand your needs and identify gaps in service as part of the laboratory's commitment to continuous quality improvement. This year, we are providing a summary of last year’s (2022 survey) results and changes initiated in response to your feedback found on the Laboratory Services Section website here: Laboratory Customer Service Survey.
I encourage you to take the 2023 Laboratory Customer Service Survey. It should not take more than 10 minutes to complete. For questions or concerns, please send an email to: Labinfo@dshs.texas.gov.
2023 Laboratory Customer Service Survey.
**Feedback will be collected until June 30, 2023,**
Posted June 1, 2023
Request of Sickle Cell Results for Student-Athletes
NCAA Policy requires sickle cell test results for all student-athletes as of August 2022.
The Texas newborn screening panel includes testing for sickle cell trait.
As a Healthcare Provider, you can do one of the following:
- Contact DSHS Laboratory Reporting Department at 512-776-7578, on behalf of the patient, to request their Newborn Screening report. This is the fastest and most efficient way for the student-athlete to obtain their sickle cell results, or
- Fax the request to DSHS Laboratory at 512-776-7533, or
- Direct the student-athlete to the DSHS webpage, “How Patients Can Request Copies of Test Results”. The athlete will need to download the Authorization to Release Laboratory Results Form. All fields are required. They will need to fax, mail, or securely email the completed Authorization to Release Laboratory Results Form and a copy of their current and valid government-issued photo ID to DSHS Laboratory.
- DSHS Lab Reporting Department fax number: 512-776-7533
- DSHS Laboratory Reporting Group, MC 1947 P.O. Box 149347 Austin, TX 78714-9347
- Email: Labinfo@dshs.texas.gov
For general questions or comments:
Call toll-free: 1-888-963-7111 ext. 7333 or for local calls 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted May 03, 2023
Do Not Hold Newborn Screening Specimens Due to Access Issues with the Web Applications
Do not hold your newborn screen specimens due to web application access issues. Submit specimens as soon as possible so there is no delay in the time-critical testing of newborns. All demographic information can be handwritten on the NBS kit. Ship dried specimen as soon as possible and within 24 hours of collection, preferably via overnight courier. We recommend keeping the yellow copy for your records and tracking when samples are sent to the lab.
DSHS is aware of the current issues with our newborn screening web application and is working diligently on a resolution.
For Questions Please Contact:
Newborn Screening Laboratory
Phone: 512-776-7333
Fax: 512-776-7157
Toll-free: 1-888-963-7111 ext. 7333
NewbornScreeningLab@dshs.texas.gov
Posted April 13, 2023
Important Information for Newborn Screening Web Application Users
An update to the Newborn Screening Web Application (Neometrics) will be implemented on Sunday, March 26, 2023. Improvements will be made to the login page, the home page, the Remote Demographic Entry module, search functions, and facility reports.
These changes will include:
- Self-service password reset capabilities.
- Facility Report page access from the home page.
- Changes to the overall look of the Remote Data Entry Form.
- Improved capability to correct/clear Fields in Remote Demographic Entry and Demographic Entry Search pages.
- Renaming of facility reports for better indication of their use.
For a full list of the changes as well as a view of the new Remote Demographic page download the flyer here.
For Information about Online Accounts or to Sign-Up Visit: Texas Department of State Health Services - Remote Data Systems
For Other Questions Please Contact:
Newborn Screening Laboratory
Phone: 512-776-7333
Fax: 512-776-7157
Toll-free: 1-888-963-7111 ext. 7333
NewbornScreeningLab@dshs.texas.gov
Posted March 24, 2023
DSHS Laboratory Website Upgrade
The Texas Department of State Health Services Website has changed platforms. As a result of these changes, there may be missing or broken links. As we work through the remaining issues, we appreciate your patience.
In the meantime, if there is any information or documents needed, please feel free to reach out to the appropriate department.
Need help?
Contact DSHS Laboratory:
Newborn Screening Inquiries: (512) 776-7333
Microbiology Inquiries: (512) 776-7599
Clinical Chemistry Inquiries: (512) 776-6236
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Microbiology Branch: Lab.Microbiology@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Posted January 26, 2023
Attention Newborn Screening Submitters
The NCAA Policy on requiring sickle cell test results has changed as of August 1, 2022.
With this change, there has been an increase in requests for Newborn Screening results.
As a Healthcare Provider, you can do one of the following:
- Contact DSHS Laboratory Reporting Department at 512-776-7578, on behalf of the patient, to request their Newborn Screening report. This is the fastest and most efficient way for the student-athlete to obtain their sickle cell results.
- Or Fax the request to DSHS Laboratory at 512-776-7533.
- Or Direct the student-athlete to the DSHS webpage, “How Patients Can Request Copies of Test Results”. The athlete will need to download the Authorization to Release Laboratory Results Form. All fields are required. They will need to fax, mail, or securely email the completed Authorization to Release Laboratory Results Form and a copy of their current and valid government-issued photo ID to DSHS Laboratory.
- DSHS Lab Reporting Department fax number: 512-776-7533
- DSHS Laboratory Reporting Group, MC 1947
P.O. Box 149347
Austin, TX 78714-9347 - Email: Labinfo@dshs.texas.gov
For questions or comments:
Call toll-free: 1-888-963-7111 ext. 7333 or for local calls 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted August 10, 2022
Newborn Screening Quality Improvement Hints – June 2022
How To Update Demographic Information On The Newborn Screening
AS A BEST PRACTICE: have parents review all demographic information before collection of the blood on the filter paper.
Why is it important not to attach a demographic form from one kit to the filter paper part of another kit?
Each Newborn Screening Kit has a unique serial number that is printed on both the demographic form and the filter paper. If a demographic form from one kit is used with the filter paper from another, there will be a delay in testing and the specimen may be rejected for testing. To avoid a delay in testing or specimen rejection, contact the DSHS Laboratory at the time of any accidents. Small demographic errors can be fixed on the original demographic form by making a single line through the incorrect information, writing the correct information above or adjacent to the error, and initialing and dating near the correct information.
Upon receipt of a shipment of newborn screening cards, inspect to verify no manufacturing errors with the kits. If there are concerns about any kits before collection, contact the DSHS Newborn Screening Laboratory at (512) 776-7333.
Download Quality Improvement Hint Volume 6-2022 for:
- Examples of Newborn Screening Kit
- Laboratory Contact Information
- Tips on how to avoid this rejection
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7
NewbornScreeningLab@dshs.texas.gov
Posted July 6, 2022
Newborn Screening Quality Improvement Hints - April 2022 Specimen Too Old to Test Upon Receipt
In 2021, over 2,000 newborn screening specimens were rejected because specimens were received at the laboratory too old to test.
Why is it important for specimens to arrive at the DSHS laboratory within thirteen days of specimen collection?
The quick delivery of specimens to the testing laboratory is imperative. It is important that submitting facilities are mindful of the time between collection and shipment for the following reasons:
- Finding and treating these disorders early can prevent serious complications, such as growth problems, developmental delays, deafness, blindness, intellectual disabilities, seizures, and sudden or early death. Some conditions may manifest with acute symptoms in the first days of life and require immediate treatment to reduce the risk of morbidity and mortality.
- Based on CLIA and the equipment manufacturer's guidelines, the DSHS NBS Laboratory established a timeframe for specimen stability from the date of specimen collection.
- Specimens not received at the DSHS Laboratory within 13 days of collection will be unsatisfactory for testing and require a recollection.
View the National Newborn Screening Timeliness Goals.
Download Quality Improvement Hint Volume 4-2022 for:
- Examples of demographic forms
- Tips on how to avoid this rejection
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted March 15, 2022
Newborn Screening Quality Improvement Hints - March 2022
Collect the First Newborn Screen Before a Transfusion - Even if the Newborn is Not 24 Hours Old
The goal of the Texas Newborn Screening Program is to identify affected babies before symptoms arise. This way they can receive appropriate treatment and lead productive and healthy lives. Transfused blood and blood products may cause inaccurate newborn screening results.
Why is it important to collect the first Newborn Screen before a transfusion?
Tests on the Newborn Screening panel can be affected by many different factors, including transfusions. Tests that will be affected by transfusions include hemoglobinopathies, galactosemia, and DNA analysis testing. The Newborn Screening Cards have a place in the demographic section to note if the specimen was collected on a newborn who had already been transfused. Transfusions can include more than red blood cells, and any kind of transfusion can impact testing. Including transfusion status information allows the newborn screening lab to accurately evaluate and report results.
Download Quality Improvement Hint Volume 3-2022 for:
- Sample of Status on the Newborn Screening Demographics form
- Guide to collection times on newborns who have been transfused
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted March 15, 2022
Newborn Screening Quality Improvement Hints - February 2022
Collect the Second Newborn Screen after 168 hours of life (7 days)
The goal of the Texas Newborn Screening Program is to identify affected babies before symptoms arise. This way they can receive appropriate treatment and lead productive and healthy lives. Collection and submission of both first and second screens improve DSHS’ ability to identify newborns who would have otherwise gone undetected.
Why is it important to collect the routine second Newborn Screen after 168 hours of life?
The Texas Department of State Health Services recommends the collection of the routine second newborn screening specimen between 7 to 14 days of age. However, specimens collected on the 7th day of life can be less than 7 days (i.e., less than 168 hours) if the time of collection is before the time of birth 7 days earlier (e.g., 6 days and 20 hours). Some DSHS screening algorithms are based on age in hours, and therefore second screens collected before 168 hours may have an increased risk to have false negative results. It is recommended that the routine second screen on a newborn be collected after 168 hours of life.
Note: When collecting a non-routine second screen, the additional screen must be collected within the time frame advised by the Clinical Care Coordination staff or the newborn screening result report.
Download Quality Improvement Hint Volume 2-2022 for:
- Examples of completed demographics
- Tips for ensuring accurate test results
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted February 10, 2022
Importance of Reporting Newborn Screening Clinical Results to the Texas Newborn Screening Program
Healthcare providers are required to report the outcome of the clinical evaluation of a baby with an out-of-range newborn screen including confirmed diagnoses and cleared babies to the DSHS Clinical Care Coordination Unit. Reporting this information is important to help ensure appropriate linkage to care and follow-up for each baby.
In the rare circumstance that a baby/child is diagnosed with a condition on the Texas newborn screening panel that was not identified by newborn screening (i.e. it was a missed case), it is required to report this to the DSHS Clinical Care Coordination staff.
Receiving diagnostic information promptly allows DSHS to review and improve newborn screening for all babies and to reduce both false positives and missed cases in the future.
We thank you for your partnership in providing the best outcomes possible for the newborn-screened population.
Texas Administrative Code 25.1.37 D Rule 37.58 provides more information about the reporting requirement.
To report confirmed, cleared, or missed cases please contact:
Newborn Screening Unit
Phone: 512-776-3957
Fax: 512-776-7450
Toll-free: 800-252-8023, ext. 3957
Posted January 28, 2022
Newborn Screening Quality Improvement Hints- January 2022
Collect the First Newborn Screen within the 24 to 48-hour time range
The Advisory Committee on Heritable Disorders in Newborns and Children Timeliness Goals state that ‘Initial NBS specimens should be collected in the appropriate time frame for the newborn’s condition but no later than 48 hours after birth’.
Why is it important to collect the first Newborn Screen after 24 hours of life?
The Texas Department of State Health Services Newborn Screening Laboratory’s recommendation for collection of the first newborn screening specimen is within 24 to 48 hours of birth. For the accurate interpretation of test results, timing of blood spot collection is very important. DSHS testing algorithms are determined down to the minute. Specimens collected outside of the 24 to 48-hour window may have different cut-off values than those collected within the ideal time frame.
There are circumstances for collection before the 24 hours, like before transfusion. For guidelines on specimen collection in special circumstances please visit /lab/nbsSpecialC.shtm.
Download Quality Improvement Hint Volume 1-2022 for:
- Examples of completed demographics
- Conversion to Military Time reference chart
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints here.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted January 18, 2022
Newborn Screening – Medicaid vs. PAID Kits
Choosing the Correct Kit for Specimen Collection
There are two types of newborn screening kits:
- Use the Insurance/Self-Pay Test Kit (form # NBS4) for newborns who are covered under a private insurance plan or for newborn screenings that are covered under a private insurance plan or will be paid for out of pocket.
- Use the Medicaid/CHIP/Charity Test Kit (form # NBS3) ONLY for newborns with Medicaid-eligible mothers, newborns covered by CHIP, or newborns who have absolutely no other payment option as required in Texas Administrative Code, Title 25 Section 37.55.
Tips for choosing and using the correct kit:
- Prior to specimen collection, verify the payor or insurance on file for the newborn’s hospital services.
- Attach the appropriate kit type to the newborn’s chart or medical record so it is available for staff who will collect the specimen.
- If the newborn does not have insurance and the mother is covered under Medicaid, collect the specimen on the NBS3 (Medicaid/CHIP/Charity) kit.
- If unsure of the newborn’s insurance or Medicaid status, collect the specimen on the NBS4 (Insurance/Self-Pay) kit. DSHS will credit the facility’s account for the cost of the kit if a Medicaid-eligible newborn has a specimen collected on an NBS4 kit.
What if a Newborn Screen was drawn on the wrong kit?
If a specimen was collected on an Insurance/Self-Pay kit but it should have been Medicaid/CHIP/Charity:
- Please submit the specimen on the kit collected. DO NOT ALTER THE KIT IN ANY WAY. DSHS performs checks to identify Medicaid-eligible specimens collected on Insurance/Self-Pay kits. If a Medicaid-eligible patient has a specimen collected on an Insurance/Self-Pay kit, the facility’s account will be credited for the cost of the kit. The reimbursement process takes 6 - 9 months.
If a specimen was collected on a Medicaid/CHIP/Charity kit, but it should have been Insurance/Self Pay:
- Please submit the specimen. Note on the test kit that the specimen should have been collected on an Insurance/Self-Pay kit. DSHS will bill the facility for the cost of the kit.
Contact the Newborn Screening Laboratory:
Phone: 1 (888) 963-7111 ext. 7333 or Locally: 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted January 07, 2022
Newborn Screening Quality Improvement Hints- December 2021
Missing Date of Birth or Collection
In 2020, over 95 newborn screening specimens were rejected because the date of collection was missing from the demographic form. All of these specimens required a recollection and caused critical delays in testing.
Why is a specimen with missing demographic information rejected?
In the laboratory, specimens are categorized based on the baby’s age at the time of specimen collection. For this reason, the date of birth and the date of collection must be recorded accurately. When the information is missing, testing may be delayed. Specimens that are missing the date of collection may be unsatisfactory for testing.
Accurate and complete demographic information is necessary for timely newborn identification and provider contact if there is an abnormal result.
Download Quality Improvement Hint Volume 6-2021 for:
- Examples of this type of rejection
- Tips on how to avoid this rejection
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints here.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted December 9, 2021
Attention Newborn Screening Submitters
Delays in shipping services seen around the holidays can increase the risk of specimens being too old to test upon receipt.
Specimens that are not received by the DSHS Laboratory within 13 days of collection will be UNSATISFACTORY for testing.
TIPS TO AVOID THIS TYPE OF REJECTION:
- Ship dried specimens as soon as possible, ideally within 24 hours. Let specimens dry in the horizontal position, on a flat non-porous surface, for 3 to 4 hours before packing.
- National recommendations state that first screen specimens should arrive at the DSHS Laboratory within 24 hours after collection.
- Keep in mind that courier and mail services may have delays during the holidays.
- DO NOT hold specimens for bulk mailing or shipping.
- Ensure all specimens have the correct name, date of birth, and date of collection before shipping. Incorrect or incomplete demographic information can lead to testing delays or rejection.
- To avoid delays, assign an individual to double check all newborn screening specimens to ensure spot quality is acceptable and all demographic information is complete before shipment
- Shipping specimens by USPS Priority or Express Mail, UPS Next Day Air, FedEx Overnight, or other types of next day shipping, can help avoid delays.
For questions or comments:
Call toll-free: 1-888-963-7111 ext. 7333 or for local calls 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted December 1, 2021
Newborn Screening Quality Improvement Hints
Spot Focus on Rejection when Blood Did Not Completely Fill Specimen Circles
In 2020, over 250 newborn screening specimens were rejected because the blood did not completely fill the specimen circles. All of these specimens required a recollection and caused critical delays to testing.
Why will a specimen that did not fill the circles be unsatisfactory for testing?
When all circles are not filled, there will not be enough blood for the laboratory to punch samples with automated equipment. Without a sufficient amount of blood, the laboratory will be unable to complete all tests; therefore, these specimens will be unsatisfactory for testing.
Download Quality Improvement Hint Volume 5-2021 for:
- Example of this type of rejection
- Tips on how to avoid this rejection
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints here.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted November 19, 2021
Newborn Screening Quality Improvement Hints
Spot Focus on Rejection when Filter Paper is Scratched from the Possible Use of Capillary Tubes
In 2020, over 180 newborn screening specimens were rejected because the filter paper was scratched from the possible use of capillary tubes. All of these specimens required a recollection and caused critical delays to testing.
Why is a specimen with filter paper that is scratched from the possible use of capillary tubes rejected?
The filter paper is designed to hold a specific amount of blood. When the filter paper has been scratched, there may be an insufficient amount of blood for proper testing. An insufficient amount of blood may cause test results to be inaccurate; therefore, these specimens will be unsatisfactory for testing.
Download Quality Improvement Hint Volume 4-2021 for:
- Examples of this type of rejection
- Tips on how to avoid this rejection
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints here.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted November 17, 2021
Newborn Screening Quality Improvement Hints
Spot Focus on Specimens that Appear Contaminated or Discolored
In 2020, over 230 newborn screening specimens were rejected because the blood appeared contaminated or discolored. All of these specimens required a recollection and caused critical delays in testing.
Why is a specimen that appeared contaminated or discolored rejected?
Contaminants can interfere with the test method and discoloration indicates an uneven application of blood on the filter paper, both can cause inaccurate test results.
Download Quality Improvement Hint Volume 3-2021 for:
- Examples of this type of rejection
- Tips on how to avoid this rejection
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints here.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted September 2, 2021
Newborn Screening Quality Improvement Hints- August 2021
Spot Focus on Caked, Clotted, or Layered Blood on Filter Paper
In 2020, over 1,651 newborn screening specimens were rejected because the blood was caked, clotted, or layered onto the filter paper. All of these specimens required a recollection and caused critical delays to testing.
Why is caked, clotted, or layered specimen rejected?
The filter paper is designed to hold a specific amount of blood. Caked, clotted, and layered blood specimens have too much blood. Specimens that have too much blood can cause falsely elevated results.
Download Quality Improvement Hint Volume 2-2021 for:
- Examples of this type of rejection
- Tips on how to avoid this rejection
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints here.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted August 3, 2021
Changes to 2021 Newborn Screening Specimen Collection Kits
A new lot of newborn screening specimen collection kits with serial numbers starting 21 (21-XXXXXXX) will be shipped out soon. The following are changes from previous kits.
Please ensure all information is complete, accurate, to the best of your knowledge, and legible before shipping specimens to DSHS. Incorrect, missing, misplaced, or invalid information can cause specimen rejection, delays in testing, and incorrect test results and reports.
Are older versions of the collection kit still acceptable?
Yes, all cards are still acceptable until the expiration date is printed on the card.
Questions?
Call: 1-888-963-7111 ext. 7333 or locally 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted July 15, 2021
Update-Newborn Screening (NBS) for X-ALD
Issues with testing instrumentation have been resolved and DSHS has resumed X-ALD testing for all specimens.
- Some specimens with delayed testing for X-ALD were sent to an outside laboratory for completion of testing and were reported as “Test Pending” for X-ALD, with a result reporting note stating, “Final report with results for this disorder to follow”.
- Once testing at the outside laboratory is complete, DSHS will send revised results with the final X-ALD screening results.
- All final results for specimens sent to the outside laboratory are expected to be reported by DSHS no later than Monday, July 12, 2021.
- For submitters receiving electronic results, revised results are expected to transmit as usual for all affected specimens.
- For submitters who receive reports by mail or by fax, revised reports with final results are expected to be sent the same way.
- Submitters who receive their results online will also get a copy by mail.
- Regular screening for X-ALD has been reinstated.
The DSHS Newborn Screening Program appreciates the dedication and commitment of healthcare providers to ensure the best possible outcomes for Texas newborns.
Questions?
Contact Newborn Screening Laboratory:
Phone: 1 (888) 963-7111 ext. 7333 or Locally: 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted July 08, 2021
Newborn Screening Quality Improvement Hints
Spot Focus on Incomplete Saturation
In 2020, over 2,100 newborn screening specimens were rejected because the blood did not properly soak through the filter paper. All of these specimens required a recollection and caused critical delays in testing.
Why is a specimen with incomplete saturation rejected?
The filter paper is designed to hold a specific amount of blood. Incomplete saturation will have too little blood for proper testing. An insufficient amount of blood may cause test results to be inaccurate; therefore, these specimens will be unsatisfactory for testing.
Download Quality Improvement Hint Volume 1-2021 for:
- Examples of this type of rejection
- Tips on how to avoid this rejection
- Links to other resources about newborn screening specimen collection
Find all Spot Focus Quality Improvement Hints here.
Questions?
1-888-963-7111 ext. 7333 or locally 512-776-7333
NewbornScreeningLab@dshs.texas.gov
Posted July 06, 2021
Attention Newborn Screening Submitters - DSHS Began SMA Testing June 1, 2021
The Department of State Health Services (DSHS) added screening for Spinal Muscular Atrophy (SMA) to the Texas Newborn Screening Panel effective June 1, 2021.
What is SMA?
Spinal Muscular Atrophy (SMA) is an inherited condition that affects the cells in the spinal cord that signal the muscles to work. Over time, the muscles get weaker and activities such as crawling, walking, sitting up, and controlling head movements become more difficult. Severe cases of SMA affect the muscles used for breathing and swallowing and can lead to early death.
Will samples received before June 1, 2021, be tested for SMA?
No, samples will not be screened retrospectively.
Will there be a fee increase?
Yes, an increase is expected and planned for January 2022. DSHS will notify stakeholders of the amount at least 90 days before the change.
Will more blood be needed to complete this test?
No additional blood is needed. Continue to collect 5 completely saturated or filled blood spots.
How common is SMA?
About 1 in 8,000 to 1 in 10,000 babies are affected by SMA.
Will the newborn screen detect all babies with SMA?
The test will identify SMA that is caused by a homozygous deletion of exon 7 in the SMN1 gene. This accounts for about 95% of all SMA cases.
Is there any treatment for SMA?
Although there is currently no cure for SMA, there are multiple FDA-approved treatments available and effective if diagnosed early.
What conditions are included on Texas Newborn Screening Panel?
The Texas panel includes 54 laboratories and two point-of-care screens. View a list of the conditions on the Texas Newborn Screening Panel.
Currently, the Texas Newborn Screening Laboratory screens for 30 core conditions and reports an additional 24 secondary conditions. With the addition of SMA, Texas will screen for 31 core conditions.
Where can I find more information on SMA?
Watch for continued updates, including list serve announcements, throughout the implementation process. Find more information on SMA at Baby’s First Test and CureSMA.
For questions or comments:
1-888-963-7111 ext. 7333 or 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted June 01, 2021
Newborn Screening SMA Testing and Result Reporting Statements Effective June 1, 2021
The Texas DSHS Newborn Screening (NBS) Laboratory will be adding Spinal Muscular Atrophy (SMA) to the Newborn Screening Panel. Thirteen new result reporting statements will be added as well as one new Unsatisfactory reporting statement to reflect the addition of SMA screening. These updates are scheduled to go into effect on June 1, 2021. A full list of all possible results is available.
Updated result report examples are available here:
- Normal: /lab/NBS/Normal-Mailer-Example.pdf
- Abnormal: /lab/NBS/Abnormal-Mailer-Example.pdf
- Unsatisfactory: /lab/NBS/Unsat-Mailer-Example.pdf
Reminder: Read screening result notes fully before taking action.
Previous notification, “Newborn Screening Result Report and Result Reporting Statements for Spinal Muscular Atrophy (SMA)” sent in April 2021, can be found here.
Questions / Comments
Call toll-free: 1-888-963-7111 ext. 7333 or for local calls 512-776-7333
Email: NewbornScreeningLab@dshs.state.tx.us
Posted May 27, 2021
IMPORTANT NOTICE: Spinal Muscular Atrophy (SMA) Scheduled Go Live Date
The Department of State Health Services (DSHS) will be adding screening for Spinal Muscular Atrophy (SMA) to the Texas Newborn Screening Panel on June 1, 2021.
What is SMA?
Spinal Muscular Atrophy (SMA) is an inherited condition that affects the cells in the spinal cord that signal the muscles to work. Over time, the muscles get weaker and activities such as crawling, walking, sitting up, and controlling head movements become more difficult. Severe cases of SMA affect the muscles used for breathing and swallowing and can lead to early death.
When will SMA be added to the Texas NBS Panel?
This addition is anticipated to begin on June 1, 2021.
Will there be a fee increase?
Yes, an increase is expected and planned for January 2022. DSHS will notify stakeholders of the amount at least 90 days before the change.
Will more blood be needed to complete this test?
No additional blood is needed. Continue to collect 5 completely saturated or filled blood spots.
How common is SMA?
About 1 in 8,000 to 1 in 10,000 babies are affected by SMA.
Will the newborn screen detect all babies with SMA?
The test will identify SMA that is caused by a homozygous deletion of exon 7 in the SMN1 gene. This accounts for about 95% of all SMA cases.
Is there any treatment for SMA?
Although there is currently no cure for SMA, there are multiple FDA-approved treatments available and effective if diagnosed early.
What conditions are included on Texas Newborn Screening Panel?
The Texas panel includes 54 laboratories and two point-of-care screens. View a list of the conditions on the Texas Newborn Screening Panel.
Currently, the Texas Newborn Screening Laboratory screens for 30 core conditions and reports an additional 24 secondary conditions. With the addition of SMA, Texas will screen for 31 core conditions.
Where can I find more information on SMA?
Watch for continued updates, including email notices, throughout the implementation process. Find more information on SMA at Baby’s First Test and CureSMA.
For questions or comments:
1-888-963-7111 ext. 7333 or 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted May 26, 2021
Newborn Screening Result Report and Result Reporting Statements for Spinal Muscular Atrophy (SMA)
The Texas Newborn Screening (NBS) Laboratory plans to begin testing for SMA due to homozygous deletion of exon 7 in SMN1 in the Summer of 2021.
SMA Results will display in an additional row on the disorder table:
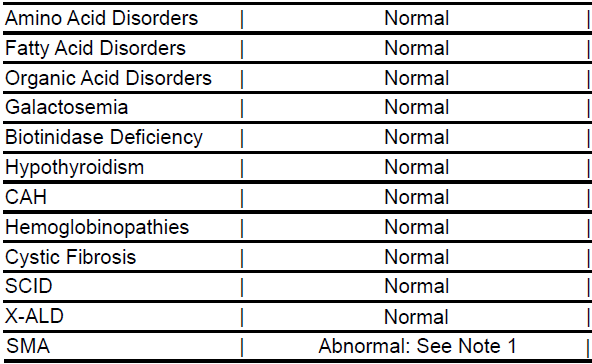
Result Reporting Statements for SMA include:
Questions? Call: 1-888-963-7111 ext. 7333 or locally 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted April, 2021
IMPORTANT NOTICE: Spinal Muscular Atrophy (SMA) to be added to the Texas Newborn Screening Panel
The Department of State Health Services (DSHS) will be adding screening for Spinal Muscular Atrophy (SMA) to the Texas Newborn Screening Panel in Summer 2021.
What is SMA?
Spinal Muscular Atrophy (SMA) is an inherited condition that affects the cells in the spinal cord that signal the muscles to work. Over time, the muscles get weaker and activities such as crawling, walking, sitting up, and controlling head movements become more difficult. Severe cases of SMA affect the muscles used for breathing and swallowing and can lead to early death.
When will SMA be added to the Texas NBS Panel?
This addition is anticipated to begin in the summer of 2021.
Will there be a fee increase?
Yes, an increase is expected and planned for January 2022. DSHS will notify stakeholders of the amount at least 90 days before the change.
Will more blood be needed to complete this test?
No additional blood is needed. Continue to collect 5 completely saturated or filled blood spots.
How common is SMA?
About 1 in 8,000 to 1 in 10,000 babies are affected by SMA.
Will the newborn screen detect all babies with SMA?
The test will identify SMA that is caused by a homozygous deletion of exon 7 in the SMN1 gene. This accounts for about 95% of all SMA cases.
Is there any treatment for SMA?
Although there is currently no cure for SMA, there are multiple FDA-approved treatments available and effective if diagnosed early.
What conditions are included on Texas Newborn Screening Panel?
The Texas panel includes 54 laboratories and two point-of-care screens. View a list of the conditions on the Texas Newborn Screening Panel.
Currently, the Texas Newborn Screening Laboratory screens for 30 core conditions and reports an additional 24 secondary conditions. With the addition of SMA, Texas will screen for 31 core conditions.
Where can I find more information on SMA?
Watch for continued updates, including list serve announcements, throughout the implementation process. Find more information on SMA at Baby’s First Test and CureSMA.
For questions or comments:
1-888-963-7111 ext. 7333 or 512-776-7333
Email: NewbornScreeningLab@dshs.texas.gov
Posted April 22, 2021