2008-2009 Texas Influenza Surveillance Activity
Current Flu Activity Report in PDF Format
Influenza Summary Report, 2008-2009 (September 28, 2008-April 11, 2009)
Background
Influenza and influenza-like illnesses (ILI) were last reportable by law in any county in Texas in 1993 (1). During that year, over 275,000 cases of influenza and influenza-like illness were reported to the Texas Department of State Health Services (DSHS) (legacy agency Texas Department of Health). The only influenza categories reportable by law in Texas for the 2008–09 season included influenza-associated pediatric fatalities, outbreaks associated with influenza, and novel influenza A infections in humans (reportable as an exotic disease). Because there is no current reporting requirement for the majority of influenza illnesses, it is unclear how many influenza-related illnesses, hospitalizations, and deaths occur each year in Texas residents. A small number of influenza cases are reported voluntarily through sentinel surveillance networks composed of laboratories, hospitals, physicians, nurses, schools, and universities located throughout the state. Additional resources include web-based influenza and ILI reporting systems, as well as local and regional health departments that gather data from surveillance participants in their jurisdictions. Data from all sources are reported to the DSHS Central Office in Austin, compiled, and presented weekly in the Texas Influenza Surveillance Report.
The national influenza reporting period begins in early October [Morbidity and Mortality Weekly Report (MMWR) week 40] and continues through late May (MMWR week 20); however, during the 2008–09 season, national influenza surveillance continued throughout the summer and early fall due to the emergence of a novel influenza A (H1N1) virus. As a result, this summary only includes data from September 28, 2008 through April 11, 2009, before the first 2009 influenza A (H1N1) pandemic viruses were identified in Texas. Data from the 2009 influenza A (H1N1) pandemic are included in the 2009–2010 influenza season summary.
Influenza surveillance in Texas continues year-round, although in reduced capacity during the summer months. The goals of influenza surveillance are to determine when and where influenza viruses are circulating, if the circulating viruses match the vaccine strains, what changes are occurring in the viruses, what impact influenza is having on hospitalizations and deaths, and the severity of influenza activity. The three main Texas influenza surveillance components are viral, morbidity, and mortality surveillance. Viral influenza surveillance at the state level consists of influenza test results reported by Texas laboratories in the National Respiratory and Enteric Virus Surveillance System (NREVSS) and specimens sent to the DSHS Austin laboratory and the Laboratory Response Network (LRN) laboratories for influenza surveillance testing. Morbidity surveillance consists of reports of novel influenza A virus infections in humans, reports of patients with ILI from Texas participants in the US Outpatient Influenza-like Illness Surveillance Network (ILINet) (formerly known as the US Influenza Sentinel Provider Surveillance Network), ILI reports submitted through local and regional health departments, and reports of influenza or ILI outbreaks. Mortality surveillance consists of reports of influenza-associated deaths in children under 18 years of age.
Overview
The 2008–09 influenza season was relatively mild in Texas and in the United States. Texas and US ILINet data indicated that the peak percentage of visits due to ILI and the number of weeks above baseline were lower in 2008–09 compared to the 2007–2008 season (2). The number of influenza-associated pediatric deaths reported in the US was 24% lower in 2008–09 compared to the previous season. Additionally, the percentage of deaths attributed to pneumonia and influenza only exceeded the US epidemic threshold for one week in the 2008–09 season compared to eight weeks in the previous season. Resistance of seasonal influenza A (H1N1) viruses to oseltamivir and influenza A (H3N2) viruses to the adamantanes (i.e., amantadine and rimantadine) was widespread during the 2008–09 season.
Viral Surveillance
National Respiratory and Enteric Virus Surveillance System (NREVSS)
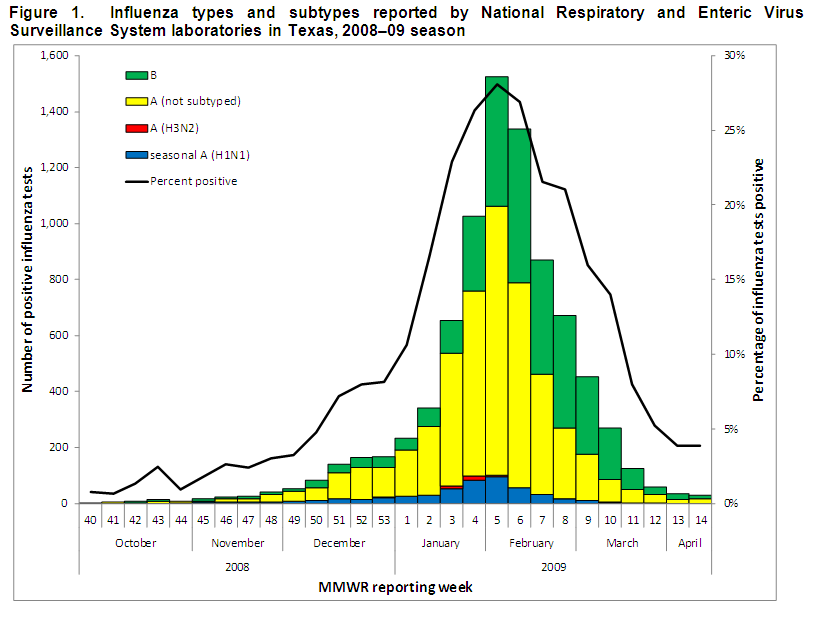
NREVSS is an online laboratory results reporting system for several respiratory and enteric viruses; the system is maintained by the Centers for Disease Control and Prevention (CDC). During the 2008–09 season, 28 participating laboratories in most Texas Health Service Regions (HSRs) submitted data on antigen detection, virus isolation (i.e., cultures), and polymerase chain reaction (PCR) testing for influenza. Of the 54,184 influenza tests that were reported to NREVSS from Texas laboratories, 8,377 (15.5%) were positive for influenza virus. Of the 8,377 positive tests, 5,277 (63.0%) tests were positive for influenza A and 3,100 (37.0%) were positive for influenza B. The majority (89.8%) of the positive test results for influenza A reported through NREVSS were reported as influenza A (not subtyped) because most laboratories in Texas do not perform subtyping. Of the 536 results for which subtyping was reported, 93.1% were identified as seasonal influenza A (H1N1) and 6.9% were identified as influenza A (H3N2). The peak of NREVSS influenza activity in Texas occurred during the week ending February 7, 2009 (MMWR week 5), when 28.1% of tests were positive for influenza virus (Figure 1).
 Texas DSHS Austin Laboratory
Texas DSHS Austin Laboratory
Influenza surveillance specimens are submitted for viral isolation to the DSHS Austin laboratory throughout the season by physicians, hospitals, clinics, and health departments across Texas.
The first influenza isolate of the season was collected on October 15, 2008 (MMWR week 42) and identified as influenza A (unable to subtype) (Figure 2). This isolate was forwarded to CDC and characterized as a “novel” influenza A (H1N1) virus of swine origin (see page 8 of this report for more information). No other swine-origin viruses were identified in Texas for the remainder of the season.
Seasonal influenza A (H1N1) was the predominant subtype of influenza A that was isolated during the 2008–09 season in Texas prior to beginning of the influenza pandemic in April 2009. Seasonal influenza A (H1N1) was first isolated in the week ending November 1, 2008 (week 44) from a resident of Bexar County. Only 37 influenza A (H3N2) viruses were isolated from specimens sent to the DSHS lab during the season; the first specimen positive for influenza A (H3N2) was reported in late December 2008. The first specimen positive for influenza B was collected during week 42 from a resident of Webb County. All three virus types and subtypes co-circulated from late December 2008 until the end of the season. Although influenza A viruses predominated during the 2008–09 season, influenza B viruses were identified more frequently for a few weeks in mid-February to early March.
 Submission of specimens for influenza surveillance began to increase during the week ending January 10, 2009 (week 1). The largest number of specimens collected for influenza surveillance, 221, occurred during the week ending February 7, 2009 (week 5), when the percentage of specimens positive for influenza was 66.5%. However, the peak percentage of specimens positive for influenza occurred during the week ending January 3, 2009 (week 53), when 70.7% of specimens were positive for influenza. This early data peak occurred because several laboratories sent culture- or PCR-confirmed influenza isolates for subtyping to DSHS Austin during this week, instead of sending untested patient specimens. The proportion of specimens positive for influenza virus in the 2008–09 season exceeded 10% for 25 weeks. Specimen submission began to decline sharply beginning in the week ending February 14, 2009 (week 6).
Submission of specimens for influenza surveillance began to increase during the week ending January 10, 2009 (week 1). The largest number of specimens collected for influenza surveillance, 221, occurred during the week ending February 7, 2009 (week 5), when the percentage of specimens positive for influenza was 66.5%. However, the peak percentage of specimens positive for influenza occurred during the week ending January 3, 2009 (week 53), when 70.7% of specimens were positive for influenza. This early data peak occurred because several laboratories sent culture- or PCR-confirmed influenza isolates for subtyping to DSHS Austin during this week, instead of sending untested patient specimens. The proportion of specimens positive for influenza virus in the 2008–09 season exceeded 10% for 25 weeks. Specimen submission began to decline sharply beginning in the week ending February 14, 2009 (week 6).
Over the course of the 2008–09 influenza season, the DSHS Austin laboratory received 1,417 specimens for influenza surveillance that met specimen testing and handling requirements; of those, 774 (54.6%) were positive for influenza virus. One of the specimens was positive for both seasonal influenza A (H1N1) and influenza B, resulting in 775 influenza viruses isolated. Of those that were positive for influenza virus, 506 (65.3%) were identified as influenza A viruses and 269 (34.7%) were identified as influenza B viruses. All influenza A isolates were subtyped: 468 (92.5%) were identified as seasonal influenza A (H1N1), 37 (7.3%) were identified as influenza A (H3N2), and 1 (0.2%) was identified as a swine-origin influenza A (H1N1) virus. The lineage was determined for 262 (97.4%) influenza B viruses: 15 (5.7%) viruses belonged to the Yamagata lineage (i.e., the lineage included in the 2008–09 Northern Hemisphere influenza vaccine) and 247 (94.3%) viruses belonged to the Victoria lineage.
Antigenic Characterization of Influenza Isolates Received by the DSHS Austin Laboratory
Like other state virology laboratories in the country, DSHS submits early, mid, and late-season as well as unusual isolates to the CDC for strain characterization. Sixty isolates were submitted to CDC during the 2008–09 season. CDC was unable to grow influenza virus from 8 of the 60 isolates that were tested, although these were subtyped by PCR testing.
From September 28, 2008 through April 11, 2009, 52 influenza viruses from Texas were antigenically characterized: 21 influenza A (H1N1) viruses, 8 influenza A (H3N2) viruses, and 23 influenza B viruses (Figure 3). Twenty (95%) of the 21 influenza A (H1N1) viruses were characterized as antigenically similar to A/Brisbane/59/2007 (H1N1), the 2008–09 Northern Hemisphere vaccine influenza A (H1N1) component. One influenza A (H1N1) virus was identified as an influenza A (H1N1) swine-origin virus. All 8 influenza A (H3N2) viruses were characterized as similar to A/Brisbane/10/2007 (H3N2), the 2008–09 Northern Hemisphere influenza A (H3N2) vaccine component.
Influenza B viruses are divided into two distinct lineages: Yamagata and Victoria. Influenza B viruses from distinct lineages provide little cross protection from infection. Of the 23 influenza B viruses characterized, 7 (30%) were characterized as similar to B/Florida/04/2006, a Yamagata lineage virus that was included in the 2008–09 Northern Hemisphere influenza vaccine. In addition, 16 (70%) B/Victoria lineage viruses were characterized; the B/Victoria lineage was not included in the 2008–09 Northern Hemisphere influenza vaccine. Two of these B/Victoria lineage viruses were similar to B/Ohio/01/2005, 10 were characterized as having reduced titers against B/Ohio/01/2005, and 4 were similar to B/Brisbane/60/2008. These results are similar to those found in the summary of all influenza isolates characterized during the 2008–09 season in the United States (2).
Antiviral Resistance Data for Influenza Isolates Received by the DSHS Austin Laboratory
Twenty-three influenza A (H1N1) viruses from Texas were tested for antiviral resistance by CDC. Of these, 22 seasonal influenza A (H1N1) viruses were resistant to oseltamivir; the swine-origin influenza A (H1N1) virus isolated in Texas in October 2008 was sensitive to oseltamivir. All 23 of these viruses were sensitive to zanamivir and the adamantanes. Seven influenza A (H3N2) viruses were tested. All were resistant to the adamantanes but were sensitive to oseltamivir and zanamivir. Twenty-three influenza B viruses were tested for antiviral resistance, and all viruses were sensitive to oseltamivir and zanamivir. (The adamantanes are not effective against influenza B viruses.)

Morbidity Surveillance
U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet)
Texas participants in ILINet report weekly on the number of patient visits for ILI by age group and the total number of patients seen for any reason. For ILINet reporting, ILI is defined as “fever (≥100°F [37.8°C], oral or equivalent) and cough and/or sore throat in the absence of a known cause other than influenza (3).” These data are used to calculate a weekly percentage of visits due to ILI.
One hundred and thirteen providers in Texas submitted data to ILINet for at least one week during the 2008–09 season (Figure 4). Data from one provider were excluded from weekly ILI calculations due to a reporting error. An average of 78 providers submitted data on an average of 20,621 patient visits each week.

The West South Central Region (Arkansas, Louisiana, Oklahoma, and Texas) ILI baseline calculated by CDC was 4.8% for the 2008–09 influenza season. The Texas baseline for the 2008–09 season was 5.47%. According to data from Texas ILINet participants, the percentage of visits due to ILI first exceeded the West South Central Region baseline during the week ending January 31, 2009 (MMWR week 4), with 5.42% of visits due to ILI (Figure 5). Influenza-like illness peaked during the week ending February 14, 2009 (week 6). During that week, ILINet providers reported that influenza-like illness accounted for 7.06% of all patient visits. The percentage of visits due to ILI fell below the regional baseline in the week ending February 28, 2009 (week 8); the percentage of visits due to ILI continued to decrease and remained below the regional baseline through week 14. Overall, ILI activity in Texas exceeded the West South Central Region baseline for four consecutive weeks. The percentage of visits due to ILI exceeded the Texas baseline for three consecutive weeks.

School Closures and Institutional Outbreaks
A total of 17 outbreaks or reports of increased absenteeism due to influenza or ILI in a school setting were reported during the 2008–09 season; five of the schools closed in response to outbreaks or high absenteeism. Schools reporting outbreaks and high absenteeism were located in HSRs 2/3, 4/5N, 7, and 8. Reports of school outbreaks and high absenteeism began during the week ending January 24, 2009 (MMWR week 3) and continued through the week ending February 14, 2009 (week 6). Nine schools reported outbreaks or high absenteeism due to ILI only. Eight schools reported outbreaks or high absenteeism due to influenza; one of the schools reported cases of influenza A (H3N2), one reported cases of influenza B, and six reported cases of influenza (type and subtype not specified).
Only one institutional outbreak due to influenza A (not subtyped) was reported in HSR 2/3 during the week ending February 7, 2009 (week 5).
Novel Influenza A Infections in Humans
One novel influenza A virus infection in a 14-year-old resident of HSR 7 was reported in Texas during the week ending October 18, 2008 (MMWR week 42). The individual’s onset of illness occurred on October 14, 2008; the duration of illness was approximately three days. Symptoms included fever, headache, cough, and conjunctivitis. The individual recovered fully without hospitalization. A specimen was collected from the patient on October 15, 2008 through routine influenza surveillance activities; the isolate was further identified by CDC as a swine-origin influenza A (H1N1) virus after the DSHS Austin laboratory could not determine the influenza A subtype. The individual reported several exposures to swine, including close contact with an ill pig, prior to illness onset. A thorough investigation determined that no other close contacts had developed illness.
Mortality Surveillance
Influenza-Associated Pediatric Mortality
Influenza-associated pediatric mortality is defined as a death resulting from an illness that is laboratory confirmed as influenza in a person under the age of 18 years (4). Eleven influenza-associated pediatric fatalities were reported to DSHS during the 2008–09 influenza season. The reported deaths occurred during the week ending January 10, 2009 (MMWR week 1) through the week ending April 11, 2009 (week 14). These 11 deaths were reported in residents of HSRs 1, 2/3, 4/5N, 6/5S, 8, 9/10, and 11. Four patients had confirmed influenza A infections and seven patients had influenza B infections. Subtyping was performed for three of the four influenza A cases, and all three viruses were identified as seasonal influenza A (H1N1).
The median age at death was 12 years, with patients ranging in age from 7 months to 17 years. Of the 11 reported cases, three patients were seven months to two years of age and eight patients were 5–17 years of age. The influenza vaccination status for the 2008–09 season was known for seven of the eleven patients; of those seven, five (71%) were not vaccinated for influenza. Nine (82%) of the eleven patients had significant underlying medical conditions and/or a bacterial co-infection. Two of the eleven patients had a methicillin-resistant Staphylococcus aureus (MRSA) co-infection.
References
- Texas Department of Health. Epidemiology in Texas 1993 Annual Report. Available
- Centers for Disease Control and Prevention. 2008–09 Influenza Season Summary. Available at http://www.cdc.gov/flu/weekly/weeklyarchives2008–09/08-09summary.htm . Accessed on December 8, 2011.
- Centers for Disease Control and Prevention. U.S. Influenza Sentinel Provider Surveillance Network: 2008–09 Workfolder. 55.20E, Rev. 06/2008
- Centers for Disease Control and Prevention. Influenza-Associated Pediatric Mortality, 2004 case definition. Available at: http://www.cdc.gov/osels/ph_surveillance/nndss/casedef/Influenza-Associated_current.htm . Accessed on December 8, 2011.
2008-2009 Texas Influenza Surveillance Information - Flu Reports
For the week ending:
10/04/08, 10/11/08, 10/18/08, 10/25/08, 11/01/08, 11/08/08, 11/15/08, 11/22/08,
11/29/08, 12/06/08, 12/13/08, 12/20/08, 12/27/08, 01/03/09, 01/10/09, 01/17/09,
01/24/09, 01/31/09, 02/07/09, 02/14/09, 02/21/09, 02/28/09, 03/07/09, 03/14/09,
03/21/09, 03/28/09, 04/04/09, 04/11/09, 04/18/09, 04/25/09, 05/02/09, 05/09/09;
05/16/09, 05/23/09, 05/30/09, 06/06/09, 06/13/09, 06/20/09, 06/27/09, 07/04/09,
07/11/09, 07/18/09, 07/25/09, 08/01/09, 08/08/09, 08/15/09, 08/22/09, 08/29/09,
09/05/09, 09/12/09, 09/19/09, 09/26/09, 10/03/09
