Laboratory Testing Services Manual - Guidelines for Specimen Shipping and Mailing
Introduction | Changes to Lab Testing Services Manual | Contact Information | Local Health Department Labs | Specimen Collection and Submission | Specimen Shipping, Mailing | Specimen Receiving, Lab Hours | Lab Tests | Forms, Fee Schedule
A phlebotomist writes patient information on a blood collection tube. Specimen labels may be printed or handwritten. If handwritten, a fine point, the permanent pen should be used to produce legible labels. Image Source: CDC/ Jim Gathany (2004)
Each year, the DSHS Laboratory in Austin receives over 1,000,000 (one million!) specimens of infectious and food-borne diseases, biological and chemical compounds, and biological agents for testing. The newborn screening program alone receives more than 800,000 newborn screening specimens each year to test newborns’ blood for 55 metabolic and genetic disorders. The laboratory also tests water from over 6,800 public water systems in Texas.* Such large volumes of specimens mean that the laboratory staff is kept busy every day, helping Texans remain safe and healthy!
An important contributing factor to specimen testing success is receiving correctly labeled, satisfactory specimens from our submitters. However, we receive specimens daily that are determined to be “unsatisfactory for testing” (unsat for short), because they were either incorrectly collected, shipped, incorrectly labeled, or missing required information. When an unsat specimen is identified, laboratory staff will notify the submitter and in certain cases request another specimen be submitted for testing. A goal of the laboratory is to optimize the submission process by providing educational resources (such as this guidance) for submitters to maximize the number of testable specimens we receive. More testable specimens arriving at the laboratory means more prompt and accurate detection and diagnosis of diseases and disorders for Texans.
This guidance was developed to address the most frequently encountered reasons for specimen unsats. Please note the information provided within is not a substitute for the training that submitters of infectious substances are required to take by law.
*While the majority of specimens received by the laboratory are submitted by healthcare facilities, drinking water samples and rabies specimens may also be submitted by the general public.
Note: The inclusion of external web links is for informational purposes only. The links do not represent an endorsement by the Texas Department of State Health Services of the goods and/or services provided by these entities. These external sites may also not be accessible to people with disabilities.
Table of Contents
- Shipping Overview: Submitter Responsibilities
- Required Training for Submitters of Hazardous/Infectious Substances
- Free Training Resources: Handling and Shipping of Infectious Substances, Category B
- Regulatory Reference Resources for Submitters of Hazardous/Infectious Substances
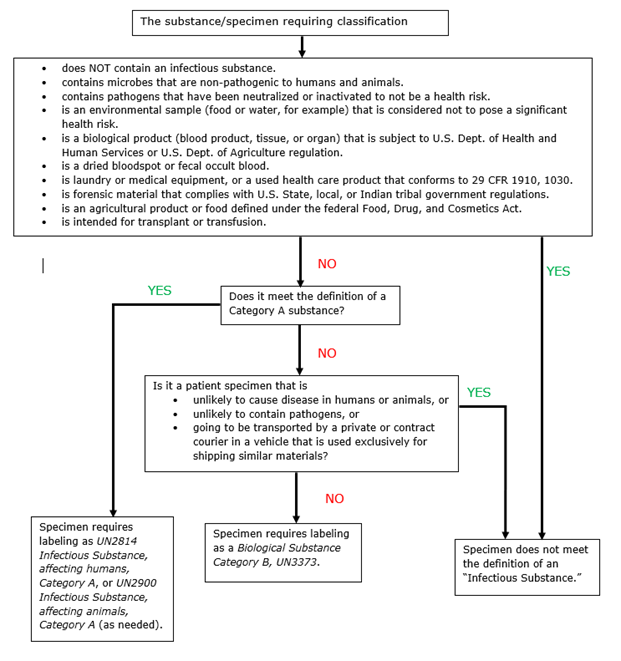
- Category A or B, or Exempt? Determining a Specimen’s Infectious Substance Classification
- Specimen Classification Decision Algorithm
- Shipping Exempt Specimens (Not Classified as Infectious Substances)
- Shipping Infectious Substances: Important Definitions to Know
- Packaging and Labeling of Category A Biological Substances
- Packaging and Labeling of Category B Biological Substances
- Providing Patient Information on Specimens and Submission Forms
- Instructions for Packing Ambient-Temperature Specimens for Shipment
- Instructions for Shipping Temperature-Sensitive Biological Substances
- Important Considerations for Submitting Biological Specimens to the Laboratory via U.S. Postal System (USPS) and Paid Courier Services
- Obtaining and Ordering Shipping Supplies from the Laboratory
- Laboratory Business Hours
- Laboratory Shipping Addresses
Shipping Overview: Submitter Responsibilities
The submission of proper specimens under optimum conditions is an important responsibility of all submitters. Please exercise care when submitting specimens and requesting tests.
The Laboratory enforces the principles of Good Laboratory Practices (GLP); a defined set of rules and criteria that ensure the reliability, reproducibility, quality, and integrity of the laboratory tests we carry out. GLP informs the laboratory’s policies, licensure, and mission, therefore, services may be withdrawn from a submitter in cases of misuse or improper specimen submission, since reliable tests cannot be performed on inadequate or substandard specimens.
Submitters have several responsibilities for the safe packing and shipping of specimens before we can reliably test them,
- Submitters should first determine the mode of transportation to be used to ship their specimen(s) to the Laboratory. The federal transportation rules that apply depend on the mode of transport used (surface: road or rail, or air freight, for example). Refer to the section Regulatory Reference Resources for Submitters of Hazardous/Infectious Substances for more details.
- Submitters must determine the correct classification of their specimen before submission. The classification of the specimen as either an “infectious substance” or as “exempt”, determines what carriers can be used to ship the specimen and how it needs to be packaged for shipping. Refer to the section Category A, Category B, or Exempt? Determining a Biological Specimen’s Classification, for details.
- Submitters who ship infectious substances are REQUIRED to receive training. Without exception, anybody who ships specimens considered Infectious Substances (Category A or Category B) is REQUIRED TO COMPLETE the appropriate training and to maintain their certification(s) for review. The shipping process can include packaging, preparing paperwork, assembling the package, labeling, or any other activity associated with the transport of packages. Training is required by the International Civil Aviation Organization (ICAO), International Air Transport Association (IATA), the U.S. Department of Transport (DOT), and the U.S. Pipeline and Hazardous Materials Safety Administration (PHMSA). Failure to properly pack and ship infectious substances materials is a violation of federal law and is punishable with fines and/or imprisonment. Refer to the section Required Training for Submitters of Hazardous/Infectious Substances.
- Submitters must pack specimens according to their classification. Submitters need to review and be knowledgeable of the federal regulatory standards for the transport of biological and infectious specimens, such as those of IATA, DOT, and PHMSA. All infectious substances must be triple-contained.
Please note: The shipping guidance provided on these pages is not a substitute for the required training. The DSHS Laboratory may provide specimen shipping guidance to submitters but cannot take responsibility for training personnel or improperly shipped specimens. Refer to sections Packaging and Labeling of Category A Biological Substances and Packaging and Labeling of Category B Biological Substances for more details on available training resources. - Submitters are responsible for shipping specimens according to all safety and labeling regulations. Submitters need to review and be knowledgeable of the federal regulatory standards for the transport of biological and infectious specimens, such as those of IATA, DOT, and PHMSA. The Laboratory may provide specimen shipping guidance to submitters but cannot take responsibility for improperly shipped specimens. Refer to the sections Regulatory Reference Resources for Submitters; Shipping Infectious Substances: Important Definitions to Know; Packaging and Labeling of Category A Biological Substances and Packaging and Labeling of Category B Biological Substances for more details/resources.
- Submitters must ensure ALL required specimen information and documentation is provided. For the laboratory to be able to test received specimens, each one must arrive with the required documentation, including being labeled correctly and accompanied by a completed specimen submission form. Use only the most recent available version of a submission form as older versions may have obsolete tests. Refer to the Guidelines for Specimen Collection and Submission sections Required Fields on Submission Forms; Sample Submission Form Showing Required Fields and Providing Patient Information on Specimens and Submission Forms to identify the required patient information and submission form information.
- Submitters must ensure specimens are shipped to the laboratory promptly. Delays in shipping specimens may result in them being too old to test when they arrive at the laboratory. Test results from old specimens are not reliable and cannot be reported.
- Submitters must ensure transport media and collection kits are not expired. The laboratory cannot test specimens that arrive on or in expired media or containers. Expired specimens will be considered unsat. Check the expiration dates on specimen collection tubes, kits, and transport media. Refer to the Specimen Collection and Submission section of this guidance for more information.
Required Training for Submitters of Hazardous/Infectious Substances
Anybody, including public health and laboratory staff involved in handling or preparing specimens for transport, the procurement of specimen couriers/carriers, and transporting specimens is required to take handling and transporting infectious substances training.
Shipping regulations and training requirements to ship infectious substances are in place to ensure infectious substances are shipped as safely as possible. Failure to properly pack and ship infectious substances materials is a violation of federal law and is punishable with fines and possibly imprisonment. The DOT/PHMSA rules that apply to the transport of infectious substances are found in the Hazardous Materials Regulations (HMR). Medical facility/provider personnel who are required to complete infectious substance training include medical and laboratory staff who are involved in the preparation of specimens that are classified as infectious substances for shipping.
Training Requirements for Shipping Infectious Substances, Category A
Anybody who ships specimens classified as Infectious Substances, Category A is REQUIRED TO COMPLETE TRAINING every two years OR if regulations change. The Category A shipping process can include packaging, preparing the paperwork, assembling the package, labeling, or any other activity associated with transport.
Shippers of Infectious Substances, Category A are bound by regulations that come from several international and national agencies. These regulations have been harmonized across agencies and are in place to ensure infectious substances are transported as safely as possible.
IATA Training resources for the shipping of Category A biological substances include:
IATA - Infectious Substances Shipping Guidelines Course (partner-taught or self-study)- Available infectious substance shipping training from IATA includes initial training and recertification classes. An IATA Certificate is awarded to participants who successfully pass the final exam.
PLEASE NOTE:
- The IATA’s training courses on handling dangerous goods and infectious substances are not free! Participants are required to purchase the coursework.
- It is the responsibility of submitters to research available Category A Infectious Substances training and to train their personnel.
- “Personnel” includes anyone who handles or prepares specimens for transport, procures specimen carriers, and or transports specimens.
- The DSHS Laboratory does not provide Category A Infectious Substances training.
Training Requirements for Shipping Infectious Substances, Category B
Per the Code of Federal Regulations, 49 CFR §173.199: Handling and Transporting Infectious Substances, personnel responsible for handling and packing Category B infectious substances must be trained on correct specimen handling and correct submission protocols. “Personnel” includes anyone who handles or prepares specimens for transport, procures specimen carriers, and or transports specimens. In addition, per IATA regulation, a dangerous goods certificate of training is required for any person who submits dangerous goods to a public carrier for transportation.
Free Training Resources: Handling and Shipping of Infectious Substances, Category B
The DSHS Emergency Preparedness Branch offers online and frequent in-person training on Shipping Infectious Biological Substances through
NOTE: DSHS online class access requires a Media Lab account; set-up instructions are found in the link https://www.dshs.texas.gov/lab/eprTraining.shtm.
U.S. Pipeline and Hazardous Materials Safety Administration (PHMSA): Hazardous Goods Handling
Hazardous Materials One-Day Workshops | PHMSA (dot.gov)
Please note that as a state agency, DSHS cannot endorse or recommend a specific vendor for training services.
Regulatory Reference Resources for Submitters of Hazardous/Infectious Substances
Additional resources on requirements for the handling and shipping of Category B infectious substances include:
U.S. Department of Transport (DOT)
International Air Transport Association (IATA)
- Dangerous Goods Regulations 63rd Edn.
U.S. Pipeline and Hazardous Materials Safety Administration (PHMSA)
PHMSA’s Check the Box Initiative (General information about shipping hazardous materials; targeted toward the general public.)

Hazardous Matt is the brand ambassador for PHMSA's Check the Box initiative, which aims to promote public awareness of the need for safer shipping of hazardous material, posters, fact sheets, brochures, and videos are available at the PHMSA's Check the Box website Source: U.S. Dot PHMSA.
Category A, Category B, or Exempt? Determining a Biological Specimen’s Classification
There are three classifications for biological specimens: Category A, Category B, and Exempt Specimen. Before submission, providers should first determine the correct classification for their biological specimens.
The Category A/Category B Infectious Substance classification criteria for transporting biological specimens were developed (adopted in 2006) to help clarify labeling and shipping requirements and to improve safety by increasing compliance. The classification requirements, based on criteria developed by the UN Committee of Experts, the World Health Organization (WHO), and the Centers for Disease Control and Prevention (CDC), among other experts, are now consistent with international standards. More details on the types of specimens classified as Category A and Category B Biological Substances may be found at USDOT PHMSA.
Important definitions that submitters of Biological Substances to the DSHS Laboratory must be knowledgeable of include
- Infectious Substance: A material known or reasonably expected to contain pathogens such as bacteria, viruses, parasites, fungi, or prions that can cause disease in humans or animals. An infectious substance may also be referred to as a Division 6.2 Infectious Substance, which is a reference to its classification in Hazardous Division 6.2 of the Hazardous Materials Table.
- Exempt Patient Specimen A material that is known, or that can be reasonably expected to not pose an infectious health risk. Exempt patient specimens can be transported in a triple-layer packaging system similar to Category A or Category B specimens, without being subject to any further infectious substance regulations. The term “Exempt human specimen” or “Exempt animal specimen” must appear on the shipping package.
- Category A: An infectious substance that is in a form that can cause permanent disability or life-threatening or fatal disease in otherwise healthy humans or animals when exposure to it occurs. Correct shipping terms include:
Category A Infectious Substances Affecting Humans, UN2814
Category A Infectious Substances Affecting Animals, UN2900
Classification as Category A must be based on the known medical history or symptoms of the source patient or animal, endemic local conditions, or a professional judgment concerning the individual circumstances of the specimen’s source human or animal. - Category B: An infectious substance not in a form generally able to cause a permanent disability or life-threatening or fatal disease in otherwise healthy humans or animals when exposure to it occurs. This includes Category B infectious substances transported for diagnostic or investigational reasons. The correct shipping name is Biological Substance, Category B, UN3373.
The PHMSA provides a list of examples of Category A Infectious Substances Affecting Humans, UN2814, and Category A Infectious Substances Affecting Animals, UN2900. The PHMSA notes the examples are provided as guidance only; the list may be subject to change.
For more information on what substances classify as Category A, refer to the most recent World Health Organization (WHO) guidance on regulations for the Transport of Infectious Substances.
Adapted from: USDOT/PHMSA , Transporting Infectious Substances Safely (2006), Federal Register.
 Shipping Exempt Specimens (Not Classified as Infectious Substances)
Shipping Exempt Specimens (Not Classified as Infectious Substances)
Several specimens tested at the DSHS Laboratory do not qualify as infectious substances because they can be reasonably expected to not pose an infectious health risk. Newborn screening (NBS) cards, for example, have dried bloodspots on them and can be reasonably expected to not contain microbial pathogens. And in specific cases, infectious substances (Category B) do not require labeling when being shipped by certain contracted courier services, which qualifies them as “exempt specimens”.
For the purpose of transport, any material defined as an “Exempt human specimen” or “Exempt animal specimen” may be transported in a triple-layer packaging system similar to those required for infectious substances, without being subject to infectious substance regulations. However, the term “Exempt human specimen” or “Exempt animal specimen” must appear on the package. In addition, there is no limit to the quantity of exempt human or animal specimens that may be carried per package, on any mode of transport. Submission and shipping guidelines for NBS specimens can be found at DSHS Newborn Screening Specimen Collection Requirements (texas.gov).
Shipping Infectious Substances: Important Definitions to Know
All specimens classified as Infectious Substances submitted to the laboratory for testing are required to be packaged in a way that minimizes the likelihood of spills and breakages and protects transportation workers, the general public, and others who may come in contact with the package while it’s in transit. Some definitions and details specific to shipping such specimens to the laboratory include
- Mailable “Infectious Substance”: A human or animal material known or reasonably expected to contain a pathogen, including but not limited to excreta (stool), secreta, blood and its components, tissue, and tissue fluids being transported for medical or veterinary use, diagnostic, or investigational purposes. All mailable infectious substances must be marked “Biological Substance, Category B, UN3373”.
Please Note: Category A infectious substances are never mailable through USPS. - Required Packaging: Specimens must be triple packaged in a way that withstands shocks, pressure changes, leaks, and other conditions related to ordinary handling in transit.
- Absorbent Material A material such as cotton balls, cellulose wadding, or paper towels that can soak up the entire contents of the specimen(s) in the primary container(s) should they break or leak.
- Primary Receptacle (Container): Must be leakproof if carrying a liquid and sift-proof if carrying a solid. Must be able to maintain its shape during shipping. Swab collection/transport tubes qualify as primary receptacles. A single primary receptacle should not contain more than 34 fluid ounces (1L) of the liquid specimen or 8.8 pounds (4kg) of the solid specimen. Two or more primary receptacles with a combined volume not exceeding 1 gallon (4 L) of liquids or 8.8 pounds (4kg) of solid may be shipped in a single secondary container.

Example of a primary receptacle: A sterile wrapped collection swab and collection tube.
Image source: DSHS AR Lab
- Secondary Container (Liner): Must be leakproof if carrying a liquid and siftproof if carrying a solid. Must be constructed of a durable material and have a means of being sealed securely. Must also contain sufficient absorbent materials to absorb the entire contents of the primary container in case of breakage or leakage. Must be labeled with the international biohazard symbol, as shown in the photo below. Biohazard symbols for Category B must not be affixed to the outer mailer.


Examples of secondary containers for shipping Category B Biological Substances. Depending on the specimen, containers may be rigid plastic such as the liner tube at left, or resealable plastic bags, as shown at right. Note the pad of absorbent material in the plastic biohazard bag. The secondary container should be labeled with a biohazard sticker. Biohazard bag photo source: ARLN Candida Swab Shipping Instructions.
- Outer Shipping Container (Mailer): Must be made of a rigid material that can withstand the knocks and bumps of transit. Must fully enclose the primary and secondary receptacles.
At least one surface must have a minimum area of 3.9 inches by 3.9 inches (100 mm by 100 mm) for appropriate labeling. The surface of the outer container should be clearly and durably marked with the required labeling.
Acceptable Outer Containers for Infectious Biological Substances
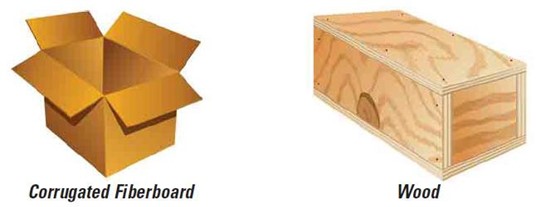

Unacceptable Outer Containers for Infectious Biological Substances

Outer packaging/mailers for shipping infectious substances must be able to withstand being dropped from a height of 1.2 meters (4 feet) without breaking. Bare polystyrene boxes, resealable plastic bags, and paper envelopes are not suitable as outer packaging.
SUBMISSION TIP
Only outer containers carrying Category A biological substances should have a biohazard notice on their exterior.
Packaging and Labeling of Category A Biological Substances
While the majority of specimens the laboratory receives are Category B Biological and Exempt Substances, occasionally Category A infectious substances are submitted for testing. Without exception, all Category A biological substances MUST be triple packaged. The secondary container must be leakproof and sealable. The outer mailer must be rigid and sturdy. If multiple fragile primary receptacles (for example, glass tubes) are placed in a single secondary receptacle, they must be individually wrapped or separated by packaging to prevent contact and reduce the likelihood of breakage. The regulatory requirements for packing Category A Biological Substances may be found at 49 CFR § 173.196.
Patient information and/or submission forms should not be affixed to the outer mailer.
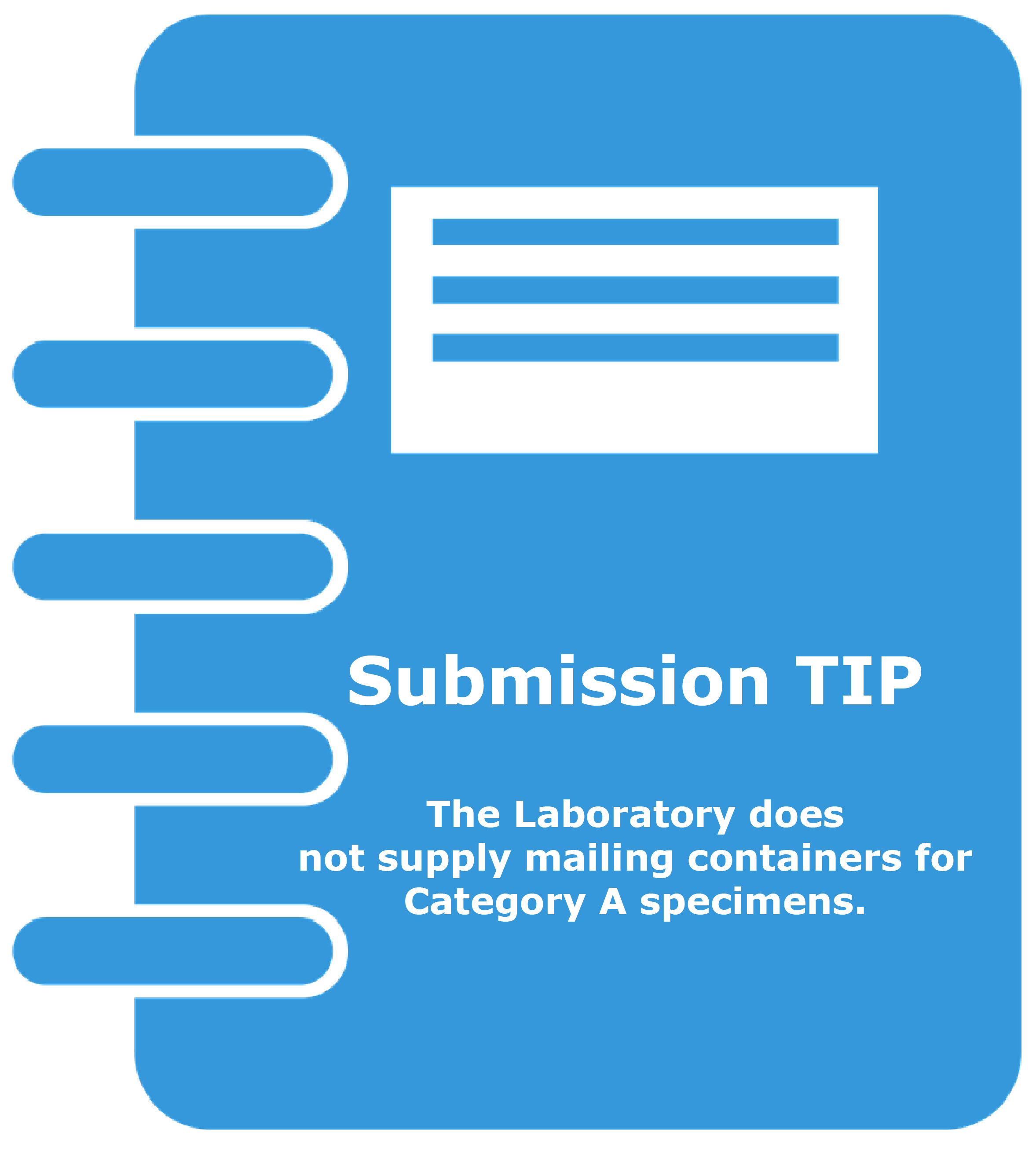
Submitters of Category A specimens must provide their shipping material since the DSHS Laboratory does not provide mailing supplies for Category A specimens.
Please note that Category A specimens should NEVER be shipped to the laboratory via the US Postal Service (USPS). The diagram below identifies the components of a correctly packaged Category A specimen.
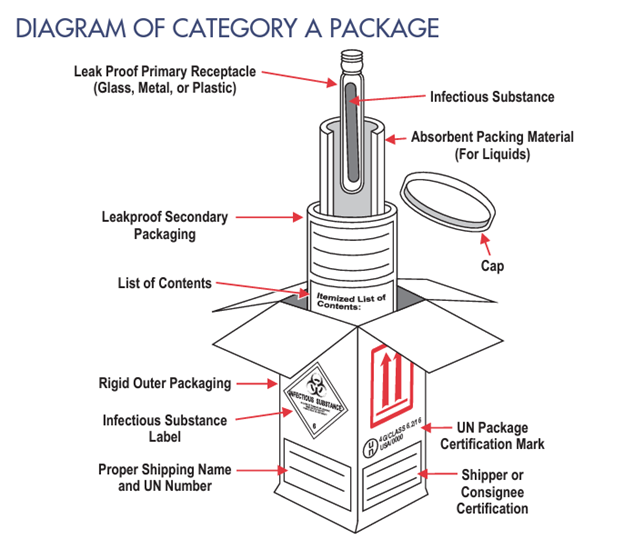
At least one surface of the outer mailer must have a minimum area of 3.9 in. x 3.9 in. (100 mm x 100 mm) for the required labels. The infectious substance/biohazard label must be displayed on the outer mailer.
Image Source: U.S. DoT PHMSA Transporting Infectious Substances Safely. https://www.phmsa.dot.gov/sites/phmsa.dot.gov/files/2020-04/Transporting-Infectious-Substances-Safely.pdf Accessed 6/14/22


Left: The required packaging and labeling for a Biological Substance Category A, infectious Substance. Note the infectious substance/ biohazard label (detail at right), affixed to the outer container. Required labels also include Category A Infectious Substances Affecting Animals, UN2900, and/or Category A Infectious Substances Affecting Humans, UN2814. If using dry ice to keep the specimen cool, a dry ice label is also required. Image source: DSHS.
Packaging and Labeling of Category B Biological Substances
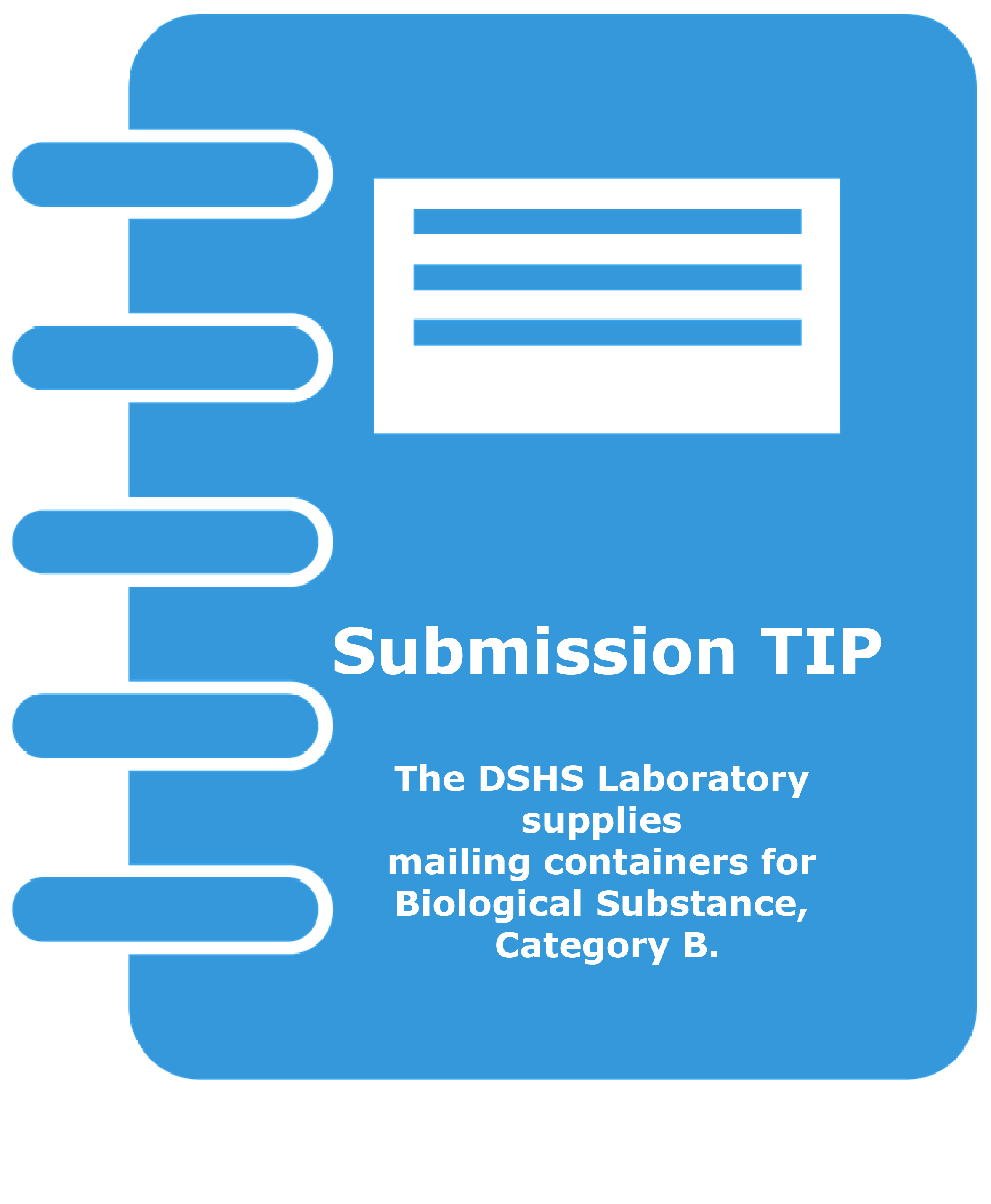
Similar to Category A, Category B substances must be triple packaged. The DSHS Laboratory supplies mailing containers for Biological Substance, Category B (UN3373) only. Use of the provided DSHS containers will ensure compliance with USPS and DOT requirements.
Please note: Due to recent shortages in the availability of secondary receptacles, the laboratory may substitute the types of rigid secondary receptacles provided to submitters.
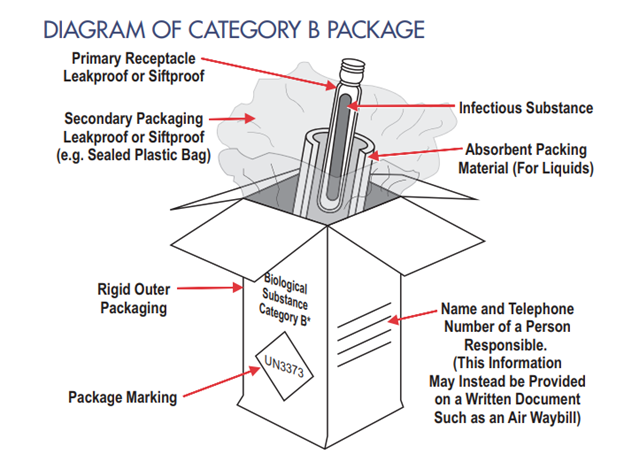
The secondary receptacle for a Category B package may be a rigid canister or a resealable plastic bag. Similar to the requirements for Category A infectious substances, at least one surface of the outer mailer must have a minimum area of 3.9 in. x 3.9 in. (100 mm x 100 mm) for the required labels. The infectious substance/biohazard label must not be displayed in the outer mailer; it should be affixed to the secondary receptacle, as shown below. Image Source: U.S. DoT PHMSA Transporting Infectious Substances Safely. https://www.phmsa.dot.gov/sites/phmsa.dot.gov/files/2020-04/Transporting-Infectious-Substances-Safely.pdf Accessed 6/14/22

Example of a secondary container for Category B Infectious Substance with a biohazard label. Image source: DSHS.


The outer mailer of a Biological Substance Category B, infectious Substance must be labeled with the UN3373 marking. Note the biohazard label is affixed to the secondary receptacle, not the exterior.
Biological Substance, Category B, UN3373 Label Specs: Each side of the diamond must be a minimum of 2 inches (50 mm) long, with a border of at least 2 mm wide. “Biological Substance Category B” and “UN3373” must be a minimum of 6 mm. Image source: DSHS.
Additional information about the specimen submission requirements for the many different tests conducted by the DSHS Laboratory may be found in the DSHS Directory of Laboratory Tests and Specimen Requirements.
Providing Patient Information on Specimens and Submission Forms
For the laboratory to be able to test received specimens, each specimen must be labeled correctly and be accompanied by a completed specimen submission form. Specimens that are incorrectly labeled cannot be tested. Good Laboratory Practice recommends, and our federal license requires that patients’ names be provided on specimen containers. Submission forms contain patients’ Protected Health Information (PHI), so they need to be packaged accordingly. To ensure the satisfactory receipt and proper testing of your specimens in the Laboratory, it is necessary to:
- Label Each Specimen Container (Primary Receptacle) The patient’s name and at least one other unique patient identifier on the specimen need to be identical to the patient identifiers on the specimen submission form(s). Acceptable specimen identifiers include, but are not limited to the:
- Patient’s Name
- Patient’s Date of Birth
- Patient’s Medical Record Number
- Specimen Identification Number
- CDC Number
- Unique Random Number
- Medicaid Number
- Newborn Screening Kit Number
Note: Location-based identifiers are NOT acceptable (e.g., hospital room number or street address)

All three patient identifiers on the specimen label match the submission form exactly.

The patient’s name on the specimen label is shortened and the ID number provided does not match the submission form. Only one patient identifier on the label and form match, so the specimen is unsatisfactory for testing.
- Provide a Separate Specimen Submission Form for Every Specimen in Container Information for multiple specimens should not be added to a submission form; one specimen, one form. Multiple submission forms may be bundled when multiple specimens are packaged together.
- Complete ALL Required Fields on Specimen Submission Forms
- Patient information on the specimen MUST match the information in the accompanying submission form.
- Use BLOCK LETTERING to complete all required information on the form. Avoid using cursive script.
- Provide payor source information, including the Medicaid number if the patient is Medicaid eligible, or if the submission is a THSteps (EPSDT) specimen.
- Provide the patient’s Name, Date of Birth, Address, the specimen’s Date of Collection, and the ICD Diagnosis Code.
- Provide the ordering physician’s National Provider Identification (NPI) number, when applicable.
- Identify the specimen source and the type of test being requested.
- Include Completed Specimen Submission Form(s) with Specimen(s) If the specimen is being shipped at ambient temperatures, affix the submission form to the outside of its secondary container. If the specimen requires shipping at frozen or chilled temperatures, first place the submission form in a sealable plastic bag to prevent it from getting wet. Then, affix the form to the lid of the insulated box (the insulated box should be shipped within an external fiberboard box.)
- Avoid Attaching Submission Forms to Outer Mailers Patient/specimen information should be attached to the internal, secondary containers only. Patients’ PHI should not be affixed to outer mailers.
Instructions for Packing Ambient-Temperature Specimens for Shipment
The number of specimen containers, such as blood collection tubes, swabs, or urine or fecal collection containers that break or leak in transit can be reduced or eliminated by using appropriate packaging and following these simple instructions.
The following directions describe packaging specimen tubes for shipment at ambient temperatures, however, all ambient temperature Category B specimens are required to be similarly packaged for shipping.
If the specimens need to be shipped at refrigeration temperatures, an insulated external mailer and cold packs/dry ice are required. Refer to Instructions for Shipping Temperature-Sensitive Biological Substances, Category B, (UN3373).

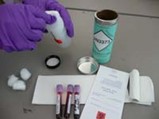





Note: Use only mailers approved for the shipping of Biological Substance, Category B, (UN3373). Use of the provided DSHS containers will ensure compliance with USPS and DOT requirements
Questions on proper packaging and shipment of specimen tubes, including blood/serum tubes should be directed to the Specimen Acquisition Branch at (512) 776-7598.
Instructions for Shipping Temperature-Sensitive Biological Substances
Several specimen types, including whole blood and serum, require shipping to the laboratory at cold temperatures. Specimens that require chilled shipping but that arrive at the lab at ambient temperature cannot be tested; they are unsatisfactory. To ensure the proper packaging of temperature-sensitive specimens:
- Identify the appropriate refrigerant for your specimen (e.g., dry ice or ice packs).
- Obtain enough refrigerant to keep specimens cold for the duration of shipping, and chilled for up to 48 hours, to account for the possibility of delays or later delivery, particularly during hot Texas summer temperatures.
- Place the frozen or chilled specimen(s) in the secondary receptacle and seal the receptacle. (Note: The photos below show Biological Substance, Category B biohazard bags. Category A specimens require hard-sided, leakproof secondary receptacles.)

- Line the bottom of the insulated outer mailer with refrigerant and place the specimens on top of it.

- Place enough refrigerant or absorbent material on top of the specimens to secure them in place.
- Secure the lid on the polystyrene box.

- Ensure the date and time the specimens were removed from the cold storage is documented on each Specimen Submission Form. Please circle “REFRIGERATOR” or “FREEZER” on the form to indicate the specimens were removed from cold storage.
- Place the completed specimen submission form(s) in a plastic sealable storage bag. Then place the plastic storage bag on top of the closed polystyrene box.
- Securely seal the outer fiberboard box with packing tape.
- Ensure a diamond-shaped UN 3373 label is affixed to the exterior of the fiberboard box when shipping Biological Substance, Category B (UN3373).
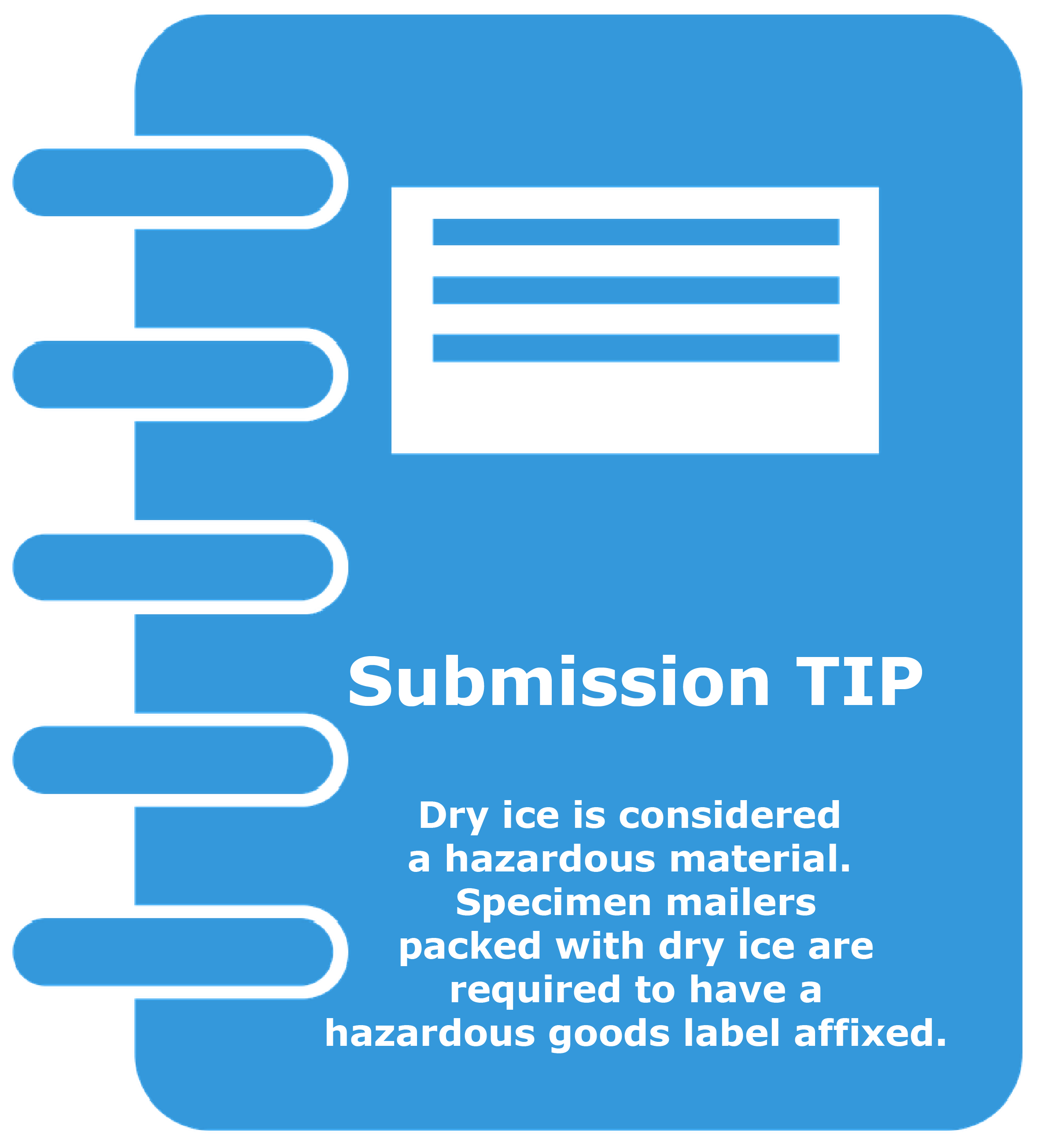
- Dry ice is considered a “dangerous good”. If shipping with dry ice:
- Use less than 5 lbs (2.25 Kg) of dry ice.
- Mark the blank box of the airbill and write “dry ice” in its Special Instructions section.
- Attach a diamond-shaped dry ice label on the package with the number “9” and “UN1845” on it. The dry ice label must include the mass of dry ice used in the container in kilograms (Kg). Ensure this information is legible and does not overlap any other label on the fiberboard box. See the graphic below, Placement of Dry Ice Label, which illustrates the placement of the dry ice label along with the other required labels and information.
- Complete all required sections of the air bill, place it inside the sleeve, and then attach it to the top of the sealed fiberboard box.
Placement of Dry Ice Label on Specimens
Biological Substance, Category B Biological Substance, Category A
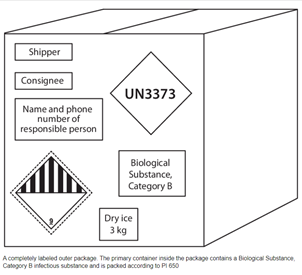
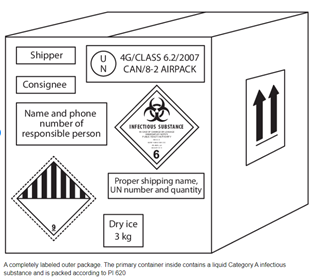
Labels Are Always Required! If shipping with dry ice, a Class 9 label, shown at the bottom right of each box, is required. The mass of dry ice (in Kg) must be provided. [Image Source: J.M. Miller et al. (2012) Guidelines for safe work practices in human and animal medical diagnostic laboratories. Recommendations of a CDC-convened, Biosafety Blue Ribbon Panel, MMWR. CDC surveillance summaries: Morbidity and mortality weekly report. CDC surveillance summaries / Centers for Disease Control 61 Suppl(1):1-102]
Special Considerations for Shipping Whole Blood and Serum Specimens
Serum that is to be shipped for testing must be stored frozen at -20°C and shipped on dry ice. The temperature during the entire shipment should remain at or below -20°C. Prepare for the potential for extremely high temperatures such as may occur in mail vans and drop boxes during a Texas summer by packing the specimens with enough refrigerant to keep them cool for at least 48 hours. Please note, be careful never to freeze whole blood specimens or serum separator tubes! Pack specimens in compliance with their classification. For assistance in determining the classification of a biological substance, refer to Category A, Category B, or Exempt? Determining a Biological Specimen’s Classification, in this section. To prevent hemolysis in whole blood specimens, pack them with shock-absorbing material to minimize the consequences of rough handling.
CAUTION!
IF USING DRY ICE, YOU MUST ENSURE THE INSULATED/POLYSTYRENE BOX IS NOT AIRTIGHT, OTHERWISE IT COULD EXPLODE AND CAUSE INJURY!
Submitting Arbovirus Surveillance Program Specimens (Live Mosquitoes)
The laboratory requires live mosquitoes for arbovirus testing. Mosquitoes are sensitive to high temperatures and low humidity; therefore, live mosquitoes should be shipped to the laboratory in the provided collection containers with several moistened paper towels to maintain high humidity and are cooled with freezer packs or plastic containers of frozen water. The collection cartons are made from pint, plastic containers with lids especially prepared for proper airflow. A corked hole is located on the side of the container. Do not use dry ice to keep the specimen cool as it sublimates to carbon dioxide gas which will asphyxiate the mosquitoes.
Specimens should be submitted to the laboratory as soon as possible after collection (preferably on the day of collection) since mortality increases with prolonged captivity. Excessive handling of specimen mosquitoes is also discouraged as it will increase the mortality rate of the mosquitoes. A G-14 Mosquito Submission form must accompany each specimen container. Attach the form to the container with a rubber band. Shipping boxes are reusable and may be used repeatedly.
For more details, visit DSHS’ Arbovirus Field Surveillance Techniques webpage, and the submission forms page to download a copy of the G-14 mosquito submission form.
Important Considerations for Submitting Biological Specimens to the Laboratory via U.S. Postal System (USPS) and Paid Courier Services
Regardless of which carrier a provider/submitter chooses to use (DHL, FedEx, LoneStar Overnight, UPS, USPS, etc.), all specimens must be packaged carefully and adequately to avoid leakage or breakage. Submitters are responsible for the proper packaging, labeling, and shipping of their specimens. The submitter is legally responsible for compliance with the shipping rules. The laboratory cannot take responsibility for improperly submitted specimens. Please note that as a state agency, DSHS does not endorse or recommend a specific shipping service.
- Many commercial carriers no longer accept biological specimens for transport. Some couriers require submitters to obtain pre-approval before shipping dangerous goods (which includes both Category A and Category B Biological Substances). Submitters are responsible for determining before shipping whether biological specimens are items prohibited by their chosen courier service.
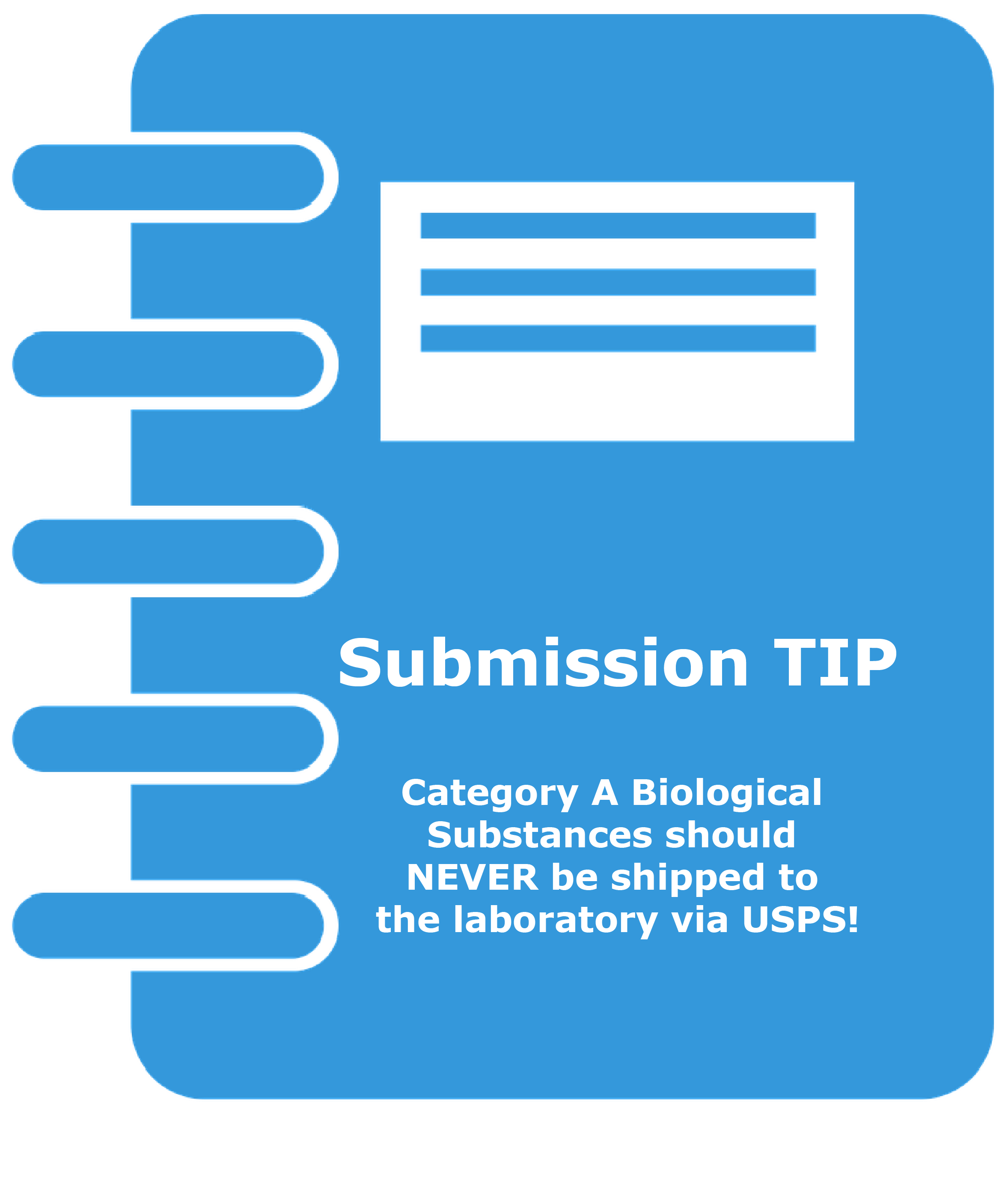
- Category A Biological Substances should NEVER be shipped via USPS! More detailed requirements for the submission of biological specimens, including Biological Substance, Category B, UN3373 through the USPS can be found at USPS.com. Shipping of Category A Biological Substance specimens requires specialized training and United Nations (UN)-approved packaging that the Laboratory does not provide.
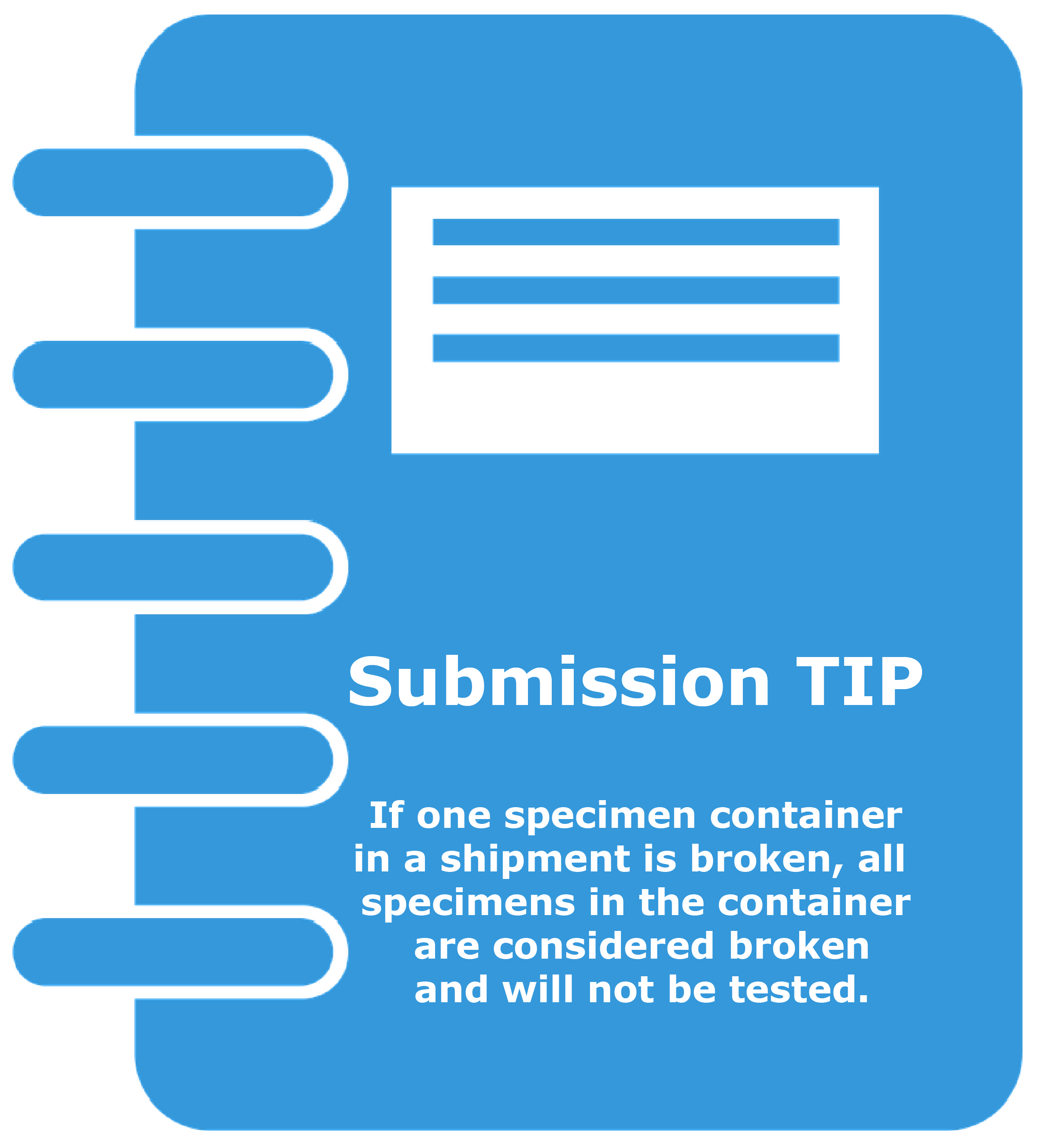
- Package specimens carefully. EVERY specimen in a container will be considered broken if even one specimen container in that secondary container is broken during shipment. For the safety of the people handling your specimens during shipping and to avoid jeopardizing the system for shipping specimens to the laboratory, always practice care and due diligence in properly securing packages for transport.
- Certain couriers can ship only ambient temperature specimens. It is the responsibility of the submitter to determine before submission whether their chosen courier shipped temperature-sensitive specimens.
- Specimens are accepted during regular business hours only.* All specimens transported through a courier will be accepted during regular business hours, which are Monday–Friday 8:00 a.m.–5:00 p.m. Routine testing of specimens delivered after 3:00 p.m. on Fridays is not carried out until the following Monday.
- Shipping overnight for arrival after closing hours, on weekends, or on holidays is discouraged. Only under exceptional circumstances can the laboratory accept deliveries outside of regular business hours. Prior approval is required for testing outside regular business hours.
- Call your overnight courier if a specimen pick-up is needed. If your chosen overnight carrier does not make regular pick-ups at your facility, you should call the carrier and let them know you need a pick-up.
- Temperature-sensitive specimens should be shipped with enough coolant to keep them chilled for 48 hours. Routine testing of specimens delivered after 3:00 p.m. on Fridays is not carried out until the following Monday. Please ensure specimens that need to be shipped cold are packed with enough refrigerant to keep them chilled until they are tested.
- Avoid shipping for weekend arrival. If a specimen is shipped for a weekend arrival, transport/courier services will hold onto it for delivery on Monday or the next regular workday, which may cause some specimens, particularly refrigerated ones, to become unsatisfactory for testing.
- Emergency Saturday testing of rabies specimens is available only with prior approval. In exceptional circumstances, and due to public health concerns, emergency weekend testing of rabies specimens can be carried out. The requirements for emergency testing of rabies specimens can be found on the Laboratory’s rabies testing webpage.
Please Note: Certain overnight delivery services offer an option for submitters to pay an additional charge for an early morning next-day specimen delivery. If the courier misses this deadline, submitters are encouraged to contact the courier to report the failure to deliver and dispute the delivery charges.
Ordering and Obtaining Shipping Supplies from the Laboratory
The laboratory provides specimen mailing containers and labels to physicians and public health laboratories upon request. The mailing containers and labels provided by the laboratory meet current Department of Transportation (DOT) and United States Postal Service (USPS) regulations for shipping Biological Substance, Category B, UN3373. Containers are the property of the State of Texas and should be used for shipping specimens to the laboratory only. Questions about setting up a submitter account to receive supplies from the laboratory and the appropriateness of specimen shipping containers may be directed to the Container Preparation Group at (512) 776-7661.
Order Forms for laboratory mailing containers and supplies may be obtained online here, or by calling the Container Preparation Group at (512) 776-7661; email ContainerPrepGroup@dshs.texas.gov. The container prep group recommends a two-month supply of shipping containers be requested each time. Orders may be faxed to (512) 776-7672. Orders will be shipped within five business days of receipt.
Mosquito/Arbovirus Surveillance Shipping Supplies
The laboratory provides both collection containers and shipping boxes for mosquito testing for arbovirus surveillance purposes. Careful handling and shipping of live mosquito specimens is essential for testing purposes. Mosquito shipping boxes are reusable and can be reused multiple times. Submitters will need to provide refrigerants such as cold packs to keep the specimens cool. Refer to Arbovirus Surveillance Program pages for further details on preparing specimens for submission.
Things to Note about Obtaining Shipping Supplies from DSHS Laboratory
- Submitters need to have an up-to-date submitter ID number and account with the Laboratory to order shipping supplies. Refer to the Guidance section Specimen Submission Process: An Overview, for more details on how to obtain a submitter ID number.
- The Laboratory supplies mailing containers for Exempt Specimens and Biological Substance, Category B specimens only.
- Use of the DSHS-provided mailing containers will ensure compliance with USPS and DOT requirements.
- Depending on the specimen type, some shipping materials, particularly secondary mailing containers and outer mailers may be reusable. Please confirm the ability to reuse containers by contacting the Specimen Acquisition group at (512) 776-7598.
- While the Laboratory accepts certain hazardous infectious specimens (Biological Substance, Category A) for testing, the laboratory does not supply the special mailing containers required for shipping Category A specimens.
- Submitters are responsible for obtaining mailing containers for Biological Substance, Category A specimens.
- Use only the most recent version of supply order forms when ordering supplies. Orders received on old forms cannot be processed.
Laboratory Business Hours
Specimens are received at the laboratory during regular business hours: Monday – Friday, 8:00 a.m. – 5:00 p.m.
Saturday delivery of rabies specimens (before 8:00 a.m.) for same-day testing is available in emergencies only. Rabies laboratory staff must first be notified about the submission by phone or email.
Toll-free Rabies Notification Number: 1-800-252-8163
Updated Electronic Rabies Specimen Submission Notification Form
Saturday or special pick up of arbovirus specimens may be arranged in emergencies only (such as an outbreak). Please call the DSHS Arbovirus Laboratory at (512) 776-7515, or (888) 963-7111 ext. 7515 (toll-free) to arrange for special pick-up and/or processing of emergency specimens.
Laboratory Shipping Addresses
For Overnight/Courier Shipping (UPS, FedEx, etc):
Texas Department of State Health Services
Laboratory Services Section
1100 W. 49th Street
Austin, TX 78756-3199
For USPS Regular and Priority Mail (Category B Infectious Substances and Exempt Specimens Only):
Texas Department of State Health Services
Laboratory Services Section, MC 1947
PO Box 149341
Austin, TX 78714-9341
For specimen shipping questions, call the Specimen Acquisition group at (512) 776-7598. For other mailing and shipping questions, call the Container Preparation Group at (512) 776-7661, or email ContainerPrepGroup@dshs.texas.gov.
